Johnson Group Research
... cleavage and functionalization of carbon-carbon bonds. While carbon-carbon single bonds are inert under a vast majority of standard reaction conditions, certain transition metal complexes promote the activation of these bonds. Research in the Johnson group will follow several avenues of study, inclu ...
... cleavage and functionalization of carbon-carbon bonds. While carbon-carbon single bonds are inert under a vast majority of standard reaction conditions, certain transition metal complexes promote the activation of these bonds. Research in the Johnson group will follow several avenues of study, inclu ...
Slides for Chapter 1-4 - Department of Chemistry and Physics
... Nucleophiles will replace the halide in C-X bonds of many alkyl halides(reaction as Lewis base) Nucleophiles that are Brønsted bases produce elimination ...
... Nucleophiles will replace the halide in C-X bonds of many alkyl halides(reaction as Lewis base) Nucleophiles that are Brønsted bases produce elimination ...
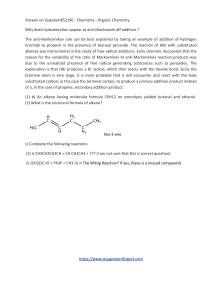
Answer on Question#52196 - Chemistry
... alkenes was instrumental in the study of free-radical additions. Early chemists discovered that the reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation ...
... alkenes was instrumental in the study of free-radical additions. Early chemists discovered that the reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation ...
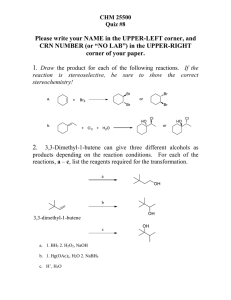
CHEM 201 Name Quiz 10 (Ch 17) ID Q1. Which of the following
... Q3. Which of the following reactions would not normally yield an alcohol? a) Oxymercuration/ demercuraction of ...
... Q3. Which of the following reactions would not normally yield an alcohol? a) Oxymercuration/ demercuraction of ...
Enantiospecific skeleton expanding cross
... pathway for the modification and expansion of α-hydroxy carbonyls, which are themselves readily available from the chiral pool. Grignard reagents possessing a range of potential substituents are reacted under mild conditions with a triflate modified carbonyl at the α-carbon to bind a wide range of s ...
... pathway for the modification and expansion of α-hydroxy carbonyls, which are themselves readily available from the chiral pool. Grignard reagents possessing a range of potential substituents are reacted under mild conditions with a triflate modified carbonyl at the α-carbon to bind a wide range of s ...
DESIGN OF CHIRAL IMINO- AND AMINOPYRIDINE LIGANDS
... The synthesis of new molecules that can work as chiral ligands in metal catalyzed enantioselective reactions is currently an area of great interest.1,2 These ligands must be capable of coordinating with the metal ion, generating around it a highly asymmetric environment so that the complex formed is ...
... The synthesis of new molecules that can work as chiral ligands in metal catalyzed enantioselective reactions is currently an area of great interest.1,2 These ligands must be capable of coordinating with the metal ion, generating around it a highly asymmetric environment so that the complex formed is ...
$doc.title
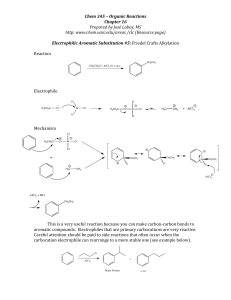
... http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
... http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
Project Details PPT
... oP R E V E N T W A S T E B Y U S I N G A SOLVENTLESS PROCESS. oM I N I M I Z E A M O U N T S O F S O L V E N T S A N D REAGENTS USED. ...
... oP R E V E N T W A S T E B Y U S I N G A SOLVENTLESS PROCESS. oM I N I M I Z E A M O U N T S O F S O L V E N T S A N D REAGENTS USED. ...
Ene reaction

The ene reaction (also known as the Alder-ene reaction) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Also,many useful Lewis acid-catalyzed ene reactions have been developed which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products.