Organic Reactions Note – Student.DOC
... The combination of two molecules to form a larger molecule by the removal of water Alcohols can be condensed to ethers using sulfuric acid A carboxylic acid and an amine can condense to from an amide ...
... The combination of two molecules to form a larger molecule by the removal of water Alcohols can be condensed to ethers using sulfuric acid A carboxylic acid and an amine can condense to from an amide ...
Preparation of alkyl halides There are lots of ways to make alkyl
... You use some kind of base in each of these cases (either triethylamine or pyridine) so that you can neutralize the acid that is formed during the reaction. The key feature of these reactions is that you are converting OH into a much better leaving group as well. 2. Preparation o ...
... You use some kind of base in each of these cases (either triethylamine or pyridine) so that you can neutralize the acid that is formed during the reaction. The key feature of these reactions is that you are converting OH into a much better leaving group as well. 2. Preparation o ...
suman_organic
... Which type of systems act as E+ or Nu-? Same system act as E+ or Nu- depending on which system it reacts. Acidity and Basicity: General idea of order of acidity and basicity. Bronsted & Lewis theory. Effect of back-bonding , aromaticity, SIR,etc. ...
... Which type of systems act as E+ or Nu-? Same system act as E+ or Nu- depending on which system it reacts. Acidity and Basicity: General idea of order of acidity and basicity. Bronsted & Lewis theory. Effect of back-bonding , aromaticity, SIR,etc. ...
Applications of Phosphorus, Sulfur, Silicon and Boron Chemistry:
... Silyl chlorides, especially bulky TBDPSCl, TIPSCl and TBDMSCl, can be used to selectively protect 1o alcohols in the presence of 2o or 3o alcohols. This can be illustrated in the following example, showing how polyfunctional molecules may be selectively manipulated with the correct protection ...
... Silyl chlorides, especially bulky TBDPSCl, TIPSCl and TBDMSCl, can be used to selectively protect 1o alcohols in the presence of 2o or 3o alcohols. This can be illustrated in the following example, showing how polyfunctional molecules may be selectively manipulated with the correct protection ...
Exam 1
... different physical properties. Boiling points are 35oC, 36oC, and 117oC, respectively. Their respective solubilities in water are 7.5g/100mL, insoluble, and 9g/100mL. (i) Draw structures for each of these compounds. (ii) Justify the observed boiling points and their solubilities. ...
... different physical properties. Boiling points are 35oC, 36oC, and 117oC, respectively. Their respective solubilities in water are 7.5g/100mL, insoluble, and 9g/100mL. (i) Draw structures for each of these compounds. (ii) Justify the observed boiling points and their solubilities. ...
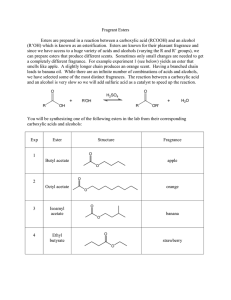
Fragrant Esters Esters are prepared in a reaction between a
... can prepare esters that produce different scents. Sometimes only small changes are needed to get a completely different fragrance. For example experiment 1 (see below) yields an ester that smells like apple. A slightly longer chain produces an orange scent. Having a branched chain leads to banana oi ...
... can prepare esters that produce different scents. Sometimes only small changes are needed to get a completely different fragrance. For example experiment 1 (see below) yields an ester that smells like apple. A slightly longer chain produces an orange scent. Having a branched chain leads to banana oi ...
Organic Tutorial 1st Year MT03
... If there is no reaction, explain why! And now some Prelim questions… 5. How can the structure of the alkyl halide influence its reactivity in SN1 and SN2 reactions? Comment on the reactivity of all of the following in nucleophilic substitution reactions. [25] ...
... If there is no reaction, explain why! And now some Prelim questions… 5. How can the structure of the alkyl halide influence its reactivity in SN1 and SN2 reactions? Comment on the reactivity of all of the following in nucleophilic substitution reactions. [25] ...
C h e m g u i d e ... ALCOHOLS: ESTERIFICATION
... 4. Esters can be also be made by reacting alcohols with acyl chlorides such as ethanoyl chloride, CH3COCl. a) Suggest a disadvantage of making, say, ethyl ethanoate using this reaction. b) What advantage(s) does the method have over the reaction between ethanol and ethanoic acid? c) Write the equati ...
... 4. Esters can be also be made by reacting alcohols with acyl chlorides such as ethanoyl chloride, CH3COCl. a) Suggest a disadvantage of making, say, ethyl ethanoate using this reaction. b) What advantage(s) does the method have over the reaction between ethanol and ethanoic acid? c) Write the equati ...
Organic Reactions Note – Student
... The combination of two molecules to form a larger molecule by the removal of water Alcohols can be condensed to ethers using sulfuric acid A carboxylic acid and an amine can condense to from an amide Esterification ...
... The combination of two molecules to form a larger molecule by the removal of water Alcohols can be condensed to ethers using sulfuric acid A carboxylic acid and an amine can condense to from an amide Esterification ...
Regulations of the International Chemistry Olympiad (IChO)
... Electrophilic addition: addition to double and triple bonds, regioselectivity (Markovnikoff’s rule), stereochemistry Electrophilic substitution: substitution on aromatic rings, influence of substituents on the reactivity and regioselectivity, electrophilic species; Elimination: E1 and E2 reactions a ...
... Electrophilic addition: addition to double and triple bonds, regioselectivity (Markovnikoff’s rule), stereochemistry Electrophilic substitution: substitution on aromatic rings, influence of substituents on the reactivity and regioselectivity, electrophilic species; Elimination: E1 and E2 reactions a ...
N H CCl3 C O N CCl3 C Cl (ii) SOCl2 7.55 g 7.78 g CCl C N NH N H
... (a) The amino acid arginine (p.1265) normally functions as a base in biological systems. Draw the structure for the side chain of arginine in its conjugate acid form. What is the approximate pKa value for the conjugate acid form of the arginine side chain? (Hint: a nitrogen atom which is part of a d ...
... (a) The amino acid arginine (p.1265) normally functions as a base in biological systems. Draw the structure for the side chain of arginine in its conjugate acid form. What is the approximate pKa value for the conjugate acid form of the arginine side chain? (Hint: a nitrogen atom which is part of a d ...
Slide 1
... One needs to consider an alternative if there is another functional group present in the compound ...
... One needs to consider an alternative if there is another functional group present in the compound ...
Preface - Wiley Online Library
... atom. Nitrogen boasts the strongest homoatomic bond rendering it chemically rather inert, but yet its fixation into plant material can be accomplished under the mildest of conditions. In his marvelous book, The Disappearing Spoon, Sean McKean eloquently summed up the idiosyncratic character of nitro ...
... atom. Nitrogen boasts the strongest homoatomic bond rendering it chemically rather inert, but yet its fixation into plant material can be accomplished under the mildest of conditions. In his marvelous book, The Disappearing Spoon, Sean McKean eloquently summed up the idiosyncratic character of nitro ...
Elimination Reactions
... Draw a mechanism and energy diagram for elimination of an alcohol under acidic conditions Explain how additions of water to an alkene and elimination of an alcohol are opposite mechanisms Describe how to shift equilibrium in favor of elimination or addition Predict the major product accordin ...
... Draw a mechanism and energy diagram for elimination of an alcohol under acidic conditions Explain how additions of water to an alkene and elimination of an alcohol are opposite mechanisms Describe how to shift equilibrium in favor of elimination or addition Predict the major product accordin ...
Chemistry Review for End of year final honors
... form water? 2H2 + O2 2H2O 3.) Calculate the number of moles of Al2O3 that are produced when 0.60 mol of Fe is produce in the following reaction: 2Al + 3FeO 3Fe + Al2O3 4.) When two substances react to form products, the reactant, which is used up in the reaction, is called ______________________ ...
... form water? 2H2 + O2 2H2O 3.) Calculate the number of moles of Al2O3 that are produced when 0.60 mol of Fe is produce in the following reaction: 2Al + 3FeO 3Fe + Al2O3 4.) When two substances react to form products, the reactant, which is used up in the reaction, is called ______________________ ...
Exam 2 Review A
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
Exam 2 Review A
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
Development of New Organic Reactions by Exploiting Sulfur
... The structures of target molecules in organic synthesis are becoming more complicated, and better functional compatibility and higher selectivity are required for the efficient synthesis of complex molecules. However, these requirements are not always fulfilled with conventional organic reactions, e ...
... The structures of target molecules in organic synthesis are becoming more complicated, and better functional compatibility and higher selectivity are required for the efficient synthesis of complex molecules. However, these requirements are not always fulfilled with conventional organic reactions, e ...
Exam 2 Review A
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
Microsoft Word - Final Exam Study Guide
... stability, elimination reactions, Zaitsev’s rule, E1 mechanism, E2 mechanism, antiperiplanar, comparing substitution and elimination mechanisms, synthesis of ethers, alcohols, and epoxides, dehydration of alcohols, carbocation rearrangements, reactions of alcohols/ethers/epoxides, multistep synthesi ...
... stability, elimination reactions, Zaitsev’s rule, E1 mechanism, E2 mechanism, antiperiplanar, comparing substitution and elimination mechanisms, synthesis of ethers, alcohols, and epoxides, dehydration of alcohols, carbocation rearrangements, reactions of alcohols/ethers/epoxides, multistep synthesi ...
CHAPTER-6 DEHYDROHALOGENATION OF ALKYL HALIDES
... Dehydration of Alcohols to form Ethers • Simple, symmetrical ethers can be formed from the intermolecular acid‐catalyzed dehydration of 1° (or methyl) alcohols (a “substitution reaction”) • 2° and 3° alcohols can’t be used because they eliminate (intramolecular dehydration) to form alkenes ...
... Dehydration of Alcohols to form Ethers • Simple, symmetrical ethers can be formed from the intermolecular acid‐catalyzed dehydration of 1° (or methyl) alcohols (a “substitution reaction”) • 2° and 3° alcohols can’t be used because they eliminate (intramolecular dehydration) to form alkenes ...
CH 3502 4500
... 17. Explain Williamson’s synthesis of ethers. 18. Discuss Norrish type-I reaction. 19. Discuss the mechanism of Wittig reaction and its uses in organic synthesis. 20. Explain Wolf-Kishner reduction with its mechanism. 21. Give any two methods of preparation of adipic acid. 22. Discuss the mechanism ...
... 17. Explain Williamson’s synthesis of ethers. 18. Discuss Norrish type-I reaction. 19. Discuss the mechanism of Wittig reaction and its uses in organic synthesis. 20. Explain Wolf-Kishner reduction with its mechanism. 21. Give any two methods of preparation of adipic acid. 22. Discuss the mechanism ...
- professional publication
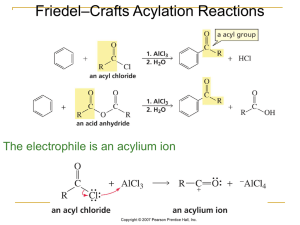
... Electrophilic Aromatic Substitutions Effect of Substituent Groups, Determination of Orientation, Determination of Relative Reactivity, Classification of Substituent Groups, Mechanism of Nitration, Sulphonation, Halogenation, Friedel Craft’s Alkylation and Friedel Craft’s Acylation, Reactivity and Or ...
... Electrophilic Aromatic Substitutions Effect of Substituent Groups, Determination of Orientation, Determination of Relative Reactivity, Classification of Substituent Groups, Mechanism of Nitration, Sulphonation, Halogenation, Friedel Craft’s Alkylation and Friedel Craft’s Acylation, Reactivity and Or ...
Organometallic Reactions and Catalysis
... – Involved a carbene complex – The carbene reacts with an alkene to form a metallocyclobutane intermediate. The intermediate can either revert to reactants or form new products. – Schrock metathesis catalysts are most effective and the most studied (available commercially). ...
... – Involved a carbene complex – The carbene reacts with an alkene to form a metallocyclobutane intermediate. The intermediate can either revert to reactants or form new products. – Schrock metathesis catalysts are most effective and the most studied (available commercially). ...
Ene reaction

The ene reaction (also known as the Alder-ene reaction) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Also,many useful Lewis acid-catalyzed ene reactions have been developed which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products.