Slide 1
... Additionally the non-bonding electron on the nitrogen would react with the electrophile. Reaction only occurs under extreme conditions. Note: That substitution is in the 3-position. ...
... Additionally the non-bonding electron on the nitrogen would react with the electrophile. Reaction only occurs under extreme conditions. Note: That substitution is in the 3-position. ...
Document
... oxygen atom of nitroarene radical anions led to an indole synthesis now named “Bartoli indole synthesis” in many textbooks monographs and papers. This de novo construction of the indole ring is now one of the most widely used syntheses of 7-substituted indoles. A laboratory accident moved Prof. Bart ...
... oxygen atom of nitroarene radical anions led to an indole synthesis now named “Bartoli indole synthesis” in many textbooks monographs and papers. This de novo construction of the indole ring is now one of the most widely used syntheses of 7-substituted indoles. A laboratory accident moved Prof. Bart ...
Efficient and Convenient Procedure for Protection of Hydroxyl
... chlorochromate (PCC) in [BPy]FeCl 4 media at moderate temperature (Scheme 3). To the best of our knowledge no report is available in the literature to carry out this transformation in RTIL. All the experimental results are summarized in Table 4. The method reported herein is fast and does not involv ...
... chlorochromate (PCC) in [BPy]FeCl 4 media at moderate temperature (Scheme 3). To the best of our knowledge no report is available in the literature to carry out this transformation in RTIL. All the experimental results are summarized in Table 4. The method reported herein is fast and does not involv ...
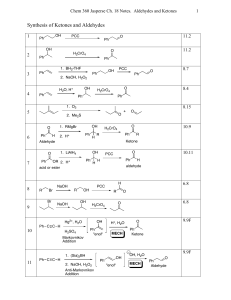
Synthesis of Ketones and Aldehydes
... 2. Remember these two reactions mainly as Markovnikov or anti-Markovnikov addition of H-OH addition to alkyne ...
... 2. Remember these two reactions mainly as Markovnikov or anti-Markovnikov addition of H-OH addition to alkyne ...
Part I Carbohydrate Auxiliaries - Wiley-VCH
... (modified Strecker reaction) are fundamental carbon–carbon bond-forming processes [3], which are efficient methods for preparing α-amino acids (Scheme 1.1). In 1987 Kunz and coworkers first reported pivaloyl protected d-galactosyl amine 3 as a very useful tool for asymmetric aminonitrile syntheses [4]. ...
... (modified Strecker reaction) are fundamental carbon–carbon bond-forming processes [3], which are efficient methods for preparing α-amino acids (Scheme 1.1). In 1987 Kunz and coworkers first reported pivaloyl protected d-galactosyl amine 3 as a very useful tool for asymmetric aminonitrile syntheses [4]. ...
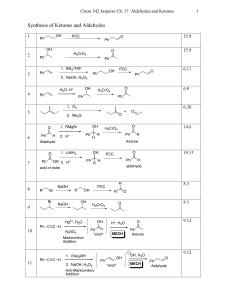
Synthesis of Ketones and Aldehydes
... 2. Remember these two reactions mainly as Markovnikov or anti-Markovnikov addition of H-OH addition to alkyne ...
... 2. Remember these two reactions mainly as Markovnikov or anti-Markovnikov addition of H-OH addition to alkyne ...
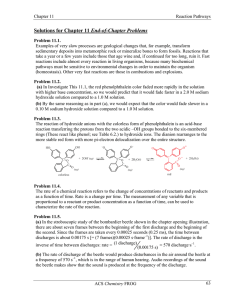
Reactions
... Alcohols can be oxidized to aldehydes or ketones using a modified form of CrO3 called PCC for pyridinium chlorochromate, (C5H6NCrO3Cl). It is a milder reagent and if you use it carefully you can stop the reaction at the intermediate ...
... Alcohols can be oxidized to aldehydes or ketones using a modified form of CrO3 called PCC for pyridinium chlorochromate, (C5H6NCrO3Cl). It is a milder reagent and if you use it carefully you can stop the reaction at the intermediate ...
Oxidation of Reduced Sulfur Species: Carbon
... The overall 0 K reaction enthalpy is computed at −176 kJ mol−1 (cf. a literature value of −175 kJ mol−1 depending on the source of the thermo data). The cis/trans reaction path degeneracy is taken into account by a symmetry factor of 2 for the hindered torsion in the trans entrance transition state ...
... The overall 0 K reaction enthalpy is computed at −176 kJ mol−1 (cf. a literature value of −175 kJ mol−1 depending on the source of the thermo data). The cis/trans reaction path degeneracy is taken into account by a symmetry factor of 2 for the hindered torsion in the trans entrance transition state ...
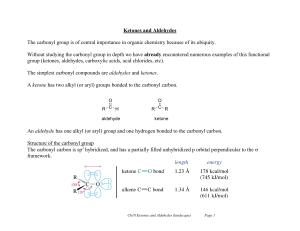
Ketones and Aldehydes
... Ketones have two alkyl substituents whereas aldehydes only have one. Carbonyl compounds undergo reaction with nucleophiles because of the polarization of the C=O bond. ...
... Ketones have two alkyl substituents whereas aldehydes only have one. Carbonyl compounds undergo reaction with nucleophiles because of the polarization of the C=O bond. ...
Esterification and Esters
... Use of Azeotropes to Remove Water. With the aliphatic alcohols and esters of medium volatility, a variety of azeotropes is encountered on distillation (see DISTILLATION, AZEOTROPIC AND EXTRACTIVE). Removal of these azeotropes from the esterification reaction mixture drives the equilibrium in favor o ...
... Use of Azeotropes to Remove Water. With the aliphatic alcohols and esters of medium volatility, a variety of azeotropes is encountered on distillation (see DISTILLATION, AZEOTROPIC AND EXTRACTIVE). Removal of these azeotropes from the esterification reaction mixture drives the equilibrium in favor o ...
High-Oxidation-State Palladium Catalysis: New Reactivity for
... and propargyl substituents, among others.[5–7] As a consequence of the carbon-rich coordination sphere of isolated PdIV complexes, C C bond formation is typically their dominant reaction. For the parent complex 2, a detailed study suggested that iodide dissociation preceded ethane formation. Hence, ...
... and propargyl substituents, among others.[5–7] As a consequence of the carbon-rich coordination sphere of isolated PdIV complexes, C C bond formation is typically their dominant reaction. For the parent complex 2, a detailed study suggested that iodide dissociation preceded ethane formation. Hence, ...
Ketones and Aldehydes Reading: Wade chapter 18, sections 18
... Dipole-dipole interactions are the main intermolecular force holding ketones and aldehydes in the liquid phase. The dipole-dipole interaction is strong because of the high dipole moment attributable to the carbonyl group, giving ketones and aldehydes higher boiling points than hydrocarbons. However, ...
... Dipole-dipole interactions are the main intermolecular force holding ketones and aldehydes in the liquid phase. The dipole-dipole interaction is strong because of the high dipole moment attributable to the carbonyl group, giving ketones and aldehydes higher boiling points than hydrocarbons. However, ...
A review of new developments in the Friedel–Crafts - Beilstein
... was discussed in the previous chapter. Even though it renders a convenient and environmental benign approach to 1,1diarylalkanes, there is still one stoichiometric side product formed during this transformation, namely water. Waste water treatment is an ongoing and expensive issue in large scale che ...
... was discussed in the previous chapter. Even though it renders a convenient and environmental benign approach to 1,1diarylalkanes, there is still one stoichiometric side product formed during this transformation, namely water. Waste water treatment is an ongoing and expensive issue in large scale che ...
Organic Chemistry Fifth Edition
... Steric factors seem to control the regioselectivity. The transition state that leads to 1-butene is less crowded than the one leading to cis or trans-2-butene. ...
... Steric factors seem to control the regioselectivity. The transition state that leads to 1-butene is less crowded than the one leading to cis or trans-2-butene. ...
ggh - Library
... showed that the Pd catalyst prepared by impregnation favoured the hydrogenation of the conjugated double bond of citral, giving citronellal as the primary hydrogenation products, whereas the amounts of unsaturated alcohols were very minor. High selectivity to citronellal was obtained for the catalys ...
... showed that the Pd catalyst prepared by impregnation favoured the hydrogenation of the conjugated double bond of citral, giving citronellal as the primary hydrogenation products, whereas the amounts of unsaturated alcohols were very minor. High selectivity to citronellal was obtained for the catalys ...
Chapter 6 Chemical Reactions
... from its red dianion is between a nucleophile (the hydroxide anion) and an electrophilic center at the middle of the dianion. Nucleophile-electrophile reactions are not always fast and this one is slowed by the fact that two negative species have to come together for the reaction to occur. Since lik ...
... from its red dianion is between a nucleophile (the hydroxide anion) and an electrophilic center at the middle of the dianion. Nucleophile-electrophile reactions are not always fast and this one is slowed by the fact that two negative species have to come together for the reaction to occur. Since lik ...
Amino Acids and Proteins
... 28.1A General Features of α-Amino Acids The 20 amino acids that occur naturally in proteins differ in the identity of the R group bonded to the α carbon. The R group is called the side chain of the amino acid. The simplest amino acid, called glycine, has R = H. All other amino acids (R ñ H) have a s ...
... 28.1A General Features of α-Amino Acids The 20 amino acids that occur naturally in proteins differ in the identity of the R group bonded to the α carbon. The R group is called the side chain of the amino acid. The simplest amino acid, called glycine, has R = H. All other amino acids (R ñ H) have a s ...
lecture 12 catalysis_transformation of alkenes_alkynes
... Metal-catalyzed exchange of alkylidene and alkylidyne units in alkenes and alkynes: ...
... Metal-catalyzed exchange of alkylidene and alkylidyne units in alkenes and alkynes: ...
Palladium(II)-Catalyzed Oxidative Cyclization Strategies Andreas K. Å. Persson
... palladium(II) readily forms π-complexes with a wide range of unsaturated hydrocarbons such as alkynes, alkenes and allenes. The coordination to palladium renders these fragments susceptible towards nucleophilic attack and/or migratory insertion. Many palladium(II)-catalyzed oxidative processes, suc ...
... palladium(II) readily forms π-complexes with a wide range of unsaturated hydrocarbons such as alkynes, alkenes and allenes. The coordination to palladium renders these fragments susceptible towards nucleophilic attack and/or migratory insertion. Many palladium(II)-catalyzed oxidative processes, suc ...
New Applications for Sulfur-Based Leaving Groups in Synthesis
... overcome these issues, the use of silicon hydrides has been developed as a relatively less-toxic alternative. One can initially assume such reagents as a poor choice for chain carriers as the Si-H bond is stronger than Sn-H. However, changing the alkyl substituents to silicon e.g. Et3SiH to (Me3Si)3 ...
... overcome these issues, the use of silicon hydrides has been developed as a relatively less-toxic alternative. One can initially assume such reagents as a poor choice for chain carriers as the Si-H bond is stronger than Sn-H. However, changing the alkyl substituents to silicon e.g. Et3SiH to (Me3Si)3 ...
Transformation of Carbon Dioxide
... formation of a methyl carbonato complex through a reaction of tin methoxide with CO2 was reported in 1967.48,49 The formation of DMC upon thermolysis of methyl (carbonato) tin was first reported in 1975, but the yield was very low; only 10% based on tin.50 Another group later reported similar result ...
... formation of a methyl carbonato complex through a reaction of tin methoxide with CO2 was reported in 1967.48,49 The formation of DMC upon thermolysis of methyl (carbonato) tin was first reported in 1975, but the yield was very low; only 10% based on tin.50 Another group later reported similar result ...
研 究 業 績 リ ス ト
... (92) Ruthenium-Catalyzed Intramolecular Cyclization of 3-Butyne-1,2-diols into Furans Y. Yada, Y. Miyake, and Y. Nishibayashi Org. Lett., to be submitted. (93) Novel Monophosphido-Bridged Diruthenium Complexes: Efficient Preparative Method and Their Catalytic Activity toward Condensation of Aldehyde ...
... (92) Ruthenium-Catalyzed Intramolecular Cyclization of 3-Butyne-1,2-diols into Furans Y. Yada, Y. Miyake, and Y. Nishibayashi Org. Lett., to be submitted. (93) Novel Monophosphido-Bridged Diruthenium Complexes: Efficient Preparative Method and Their Catalytic Activity toward Condensation of Aldehyde ...
Full-Text PDF
... allows for milder reaction conditions [5], allowing the synthesis of structurally-complex epoxides. Transition Metal (TM) complexes are very important catalysts in the epoxidation of simple alkenes and other olefinic materials, such as allylic alcohols using H2 O2 or TBHP as primary oxygen sources. ...
... allows for milder reaction conditions [5], allowing the synthesis of structurally-complex epoxides. Transition Metal (TM) complexes are very important catalysts in the epoxidation of simple alkenes and other olefinic materials, such as allylic alcohols using H2 O2 or TBHP as primary oxygen sources. ...
Ene reaction

The ene reaction (also known as the Alder-ene reaction) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Also,many useful Lewis acid-catalyzed ene reactions have been developed which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products.