university of london thesis
... The chapter covering results and discussion opens with a brief overview o f the investigational work previously carried out into the Sn I like ring opening o f a model molecule, 1-methylcyclohexene oxide. This is followed by a description o f how a new methodology for the acidic ring opening o f epo ...
... The chapter covering results and discussion opens with a brief overview o f the investigational work previously carried out into the Sn I like ring opening o f a model molecule, 1-methylcyclohexene oxide. This is followed by a description o f how a new methodology for the acidic ring opening o f epo ...
synthetic approaches for quinoline and isoquinoline
... S.N. PANDEYA, ALKA TYAGI Department of Pharmaceutical Chemistry, S.I.T.M, LKO, India Received: 10 March 2011, Revised and Accepted: 11 April 2011 ABSTRACT The Quinoline and Isoquinoline nucleus is found to be very important in pharmacy field. In recent years, a lot of synthe ...
... S.N. PANDEYA, ALKA TYAGI Department of Pharmaceutical Chemistry, S.I.T.M, LKO, India Received: 10 March 2011, Revised and Accepted: 11 April 2011 ABSTRACT The Quinoline and Isoquinoline nucleus is found to be very important in pharmacy field. In recent years, a lot of synthe ...
Chapter 15
... added and one of the oxygens is lost as HO- or CH3O- to give a primary alcohol. Since aldehydes are more easily reduced (we will learn why in a later chapter) than esters or carboxylic acids, we cannot isolate the aldehyde intermediate. ...
... added and one of the oxygens is lost as HO- or CH3O- to give a primary alcohol. Since aldehydes are more easily reduced (we will learn why in a later chapter) than esters or carboxylic acids, we cannot isolate the aldehyde intermediate. ...
Hein and Arena - faculty at Chemeketa
... In the presence of excess alcohol and a strong acid such as dry HCl, aldehydes or hemiacetals react with a second molecule of the alcohol to yield an acetal. ...
... In the presence of excess alcohol and a strong acid such as dry HCl, aldehydes or hemiacetals react with a second molecule of the alcohol to yield an acetal. ...
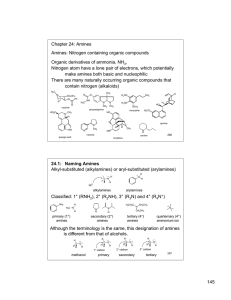
145 Chapter 24: Amines Amines: Nitrogen containing organic
... Synthesis of primary amines from the reaction of alkyl halides or tosylates with “ammonia equivalents” Azide ion is a very strong nucleophile and react with 1° or 2° alkyl halides or tosylates via an SN2 reaction. The resulting azide can be reduced to a 1° amine. H N N N ...
... Synthesis of primary amines from the reaction of alkyl halides or tosylates with “ammonia equivalents” Azide ion is a very strong nucleophile and react with 1° or 2° alkyl halides or tosylates via an SN2 reaction. The resulting azide can be reduced to a 1° amine. H N N N ...
Synthetic applications of ortho esters
... In contrast to acetal derivatives of carbonyl compounds, ortho esters have found surprisingly limited use in organic synthesis [1]. Since ortho esters are among the few carboxylic acid protective groups that demonstrate a high level of stability toward strong nucleophiles and bases, most current app ...
... In contrast to acetal derivatives of carbonyl compounds, ortho esters have found surprisingly limited use in organic synthesis [1]. Since ortho esters are among the few carboxylic acid protective groups that demonstrate a high level of stability toward strong nucleophiles and bases, most current app ...
Naming Aldehydes & Ketones
... IUPAC Rules for Naming Aldehydes 3. Form the parent aldehyde name by dropping the –e from the corresponding alkane name and adding the suffix –al. 4. Other groups attached to the parent chain are named and numbered as we ...
... IUPAC Rules for Naming Aldehydes 3. Form the parent aldehyde name by dropping the –e from the corresponding alkane name and adding the suffix –al. 4. Other groups attached to the parent chain are named and numbered as we ...
13: Carbonyl Compounds: Ketones, Aldehydes, Carboxylic Acids
... Details of Oxidation Number Calculations. The rules used to calculate these numbers are similar to those used for inorganic molecules. The H atoms are assigned an oxidation number of +1 when they are bonded to C or to more electronegative atoms. In contrast, oxygen atoms, whether singly or doubly bo ...
... Details of Oxidation Number Calculations. The rules used to calculate these numbers are similar to those used for inorganic molecules. The H atoms are assigned an oxidation number of +1 when they are bonded to C or to more electronegative atoms. In contrast, oxygen atoms, whether singly or doubly bo ...
Topic 10 SL Mark Scheme Past exam paper questions
... Tables and/or flow charts summarising all the reactions (including reagents) on organic compounds which you have met, as summarised in the scheme below. Note: There are occasions where this duplicates parts of the organic notes above. In that case, the reactions will be most useful for helping to an ...
... Tables and/or flow charts summarising all the reactions (including reagents) on organic compounds which you have met, as summarised in the scheme below. Note: There are occasions where this duplicates parts of the organic notes above. In that case, the reactions will be most useful for helping to an ...
Reactions of Alkenes: Addition Reactions
... The second step of the mechanism is the same kind of rapid carbocation–anion combination that we saw earlier as the last step in the mechanism of the reaction of alcohols with hydrogen halides (Section 4.8). This general mechanism is called electrophilic addition. It is triggered by the acid acting ...
... The second step of the mechanism is the same kind of rapid carbocation–anion combination that we saw earlier as the last step in the mechanism of the reaction of alcohols with hydrogen halides (Section 4.8). This general mechanism is called electrophilic addition. It is triggered by the acid acting ...
Synthesis of Natural Products and Related Compounds using Enyne
... available. Complexes 1b and 1c are stable and easy to handle. Thus, many researchers can use these catalysts, and various cyclic compounds have been synthesized from dienes using ring-closing metathesis (RCM). In 1999, Herrmann,[5] Nolan[6] and Grubbs[7] found the novel ruthenium carbene complexes 1 ...
... available. Complexes 1b and 1c are stable and easy to handle. Thus, many researchers can use these catalysts, and various cyclic compounds have been synthesized from dienes using ring-closing metathesis (RCM). In 1999, Herrmann,[5] Nolan[6] and Grubbs[7] found the novel ruthenium carbene complexes 1 ...
Amines
... Typical reagents : LiAlH4 / ether solvent, followed by aqueous work-up. Note that this reaction is different to that of other C=O compounds which reduce to alcohols (for example esters) The nature of the amine obtained depends on the substituents present on the original amide. Look at the N substitu ...
... Typical reagents : LiAlH4 / ether solvent, followed by aqueous work-up. Note that this reaction is different to that of other C=O compounds which reduce to alcohols (for example esters) The nature of the amine obtained depends on the substituents present on the original amide. Look at the N substitu ...
Alkenes notes
... attached to different groups, then two different structures arise which cannot be interconverted. This is known as E-Z isomerism Stereoisomers are molecules with the same molecular formula and the same arrangement of covalent bonds but with different spatial orientations of the groups. E-Z stereoiso ...
... attached to different groups, then two different structures arise which cannot be interconverted. This is known as E-Z isomerism Stereoisomers are molecules with the same molecular formula and the same arrangement of covalent bonds but with different spatial orientations of the groups. E-Z stereoiso ...
Research in our group encompasses the following 4 areas in
... monoarylation by allylic substitution and subsequent site-selective second arylation by directed allylic C-H activation giving stereoselectively anti--(aryl,styryl)-β-hydroxy acids. Presence of O2 was crucial for the second arylation via Pd(II) catalysis. ...
... monoarylation by allylic substitution and subsequent site-selective second arylation by directed allylic C-H activation giving stereoselectively anti--(aryl,styryl)-β-hydroxy acids. Presence of O2 was crucial for the second arylation via Pd(II) catalysis. ...
Full-Text PDF
... mol % of tetrakis(acetonitrile)copper(I) triflate was able to efficiently catalyze the transformation of phenol 1 to the corresponding phenyl acetate 2 in 3 min and in quantitative yield. the model substrate and acetic anhydride (4 equivalents) as the acetylation agent, we found that 1 the model ...
... mol % of tetrakis(acetonitrile)copper(I) triflate was able to efficiently catalyze the transformation of phenol 1 to the corresponding phenyl acetate 2 in 3 min and in quantitative yield. the model substrate and acetic anhydride (4 equivalents) as the acetylation agent, we found that 1 the model ...
Grignard-syn-12-ques
... A Grignard reagent, RMgX, is a very strong base and a good nucleophile; water/moisture MUST be avoided in its reaction. ...
... A Grignard reagent, RMgX, is a very strong base and a good nucleophile; water/moisture MUST be avoided in its reaction. ...
Chapter 7 Hydrosilylation of Carbon
... Hydrosilylation of Styrenes Palladium-catalyzed hydrosilylation of styrene derivatives usually proceeds with high regioselectivity to produce benzylic silanes, 1-aryl-1-silylethanes, due to the participation of π-benzylic palladium intermediates [1, 2]. It is known that bisphosphine-palladium comple ...
... Hydrosilylation of Styrenes Palladium-catalyzed hydrosilylation of styrene derivatives usually proceeds with high regioselectivity to produce benzylic silanes, 1-aryl-1-silylethanes, due to the participation of π-benzylic palladium intermediates [1, 2]. It is known that bisphosphine-palladium comple ...
BSA - Sigma
... is readily lost from the transition state during reaction, but possesses sufficient chemical stability in combination with the alkyl silyl group to allow long term storage of the derivatizing agent for use as required. As the formation of the transition state is reversible, the derivatization will o ...
... is readily lost from the transition state during reaction, but possesses sufficient chemical stability in combination with the alkyl silyl group to allow long term storage of the derivatizing agent for use as required. As the formation of the transition state is reversible, the derivatization will o ...
Reactions You Should Know When You Begin Organic II
... Usefull for ethane, propane, and isobutane only. Others give multiple products. ...
... Usefull for ethane, propane, and isobutane only. Others give multiple products. ...
C1 polymerization and related C-C bond forming - UvA-DARE
... etc.), solvent resistance, miscibility with other polymers and rheological properties.1 Thus the search for the ‘holy-grail’ in TM catalyzed polymerization continues, but it is questionable if general strategies for TM catalyzed stereospecific polymerization of polar functionalized C2 monomers can b ...
... etc.), solvent resistance, miscibility with other polymers and rheological properties.1 Thus the search for the ‘holy-grail’ in TM catalyzed polymerization continues, but it is questionable if general strategies for TM catalyzed stereospecific polymerization of polar functionalized C2 monomers can b ...
Dicyanomethylenedihydrofuran photorefractive materials
... from the electronegative trifluoromethyl group in 33, the quaternary carbon next to carbonyl is electropositive, which results in more electron transfer from nitrogen to carbonyl and the carbonyl here is then more electronegative and thus less electrophilic than the carbonyl in 30 (more shielded as ...
... from the electronegative trifluoromethyl group in 33, the quaternary carbon next to carbonyl is electropositive, which results in more electron transfer from nitrogen to carbonyl and the carbonyl here is then more electronegative and thus less electrophilic than the carbonyl in 30 (more shielded as ...
Isomeric Product Detection in the
... products is attributed to the lack of tertiary carbons in octacosane. Zhang et al.13 showed that the heterogeneous reaction of OH with cholestane (a C27H48 solid cyclic alkane with a branched aliphatic side chain) yielded few ring-opening oxidation products. This is in contrast to gas phase reaction ...
... products is attributed to the lack of tertiary carbons in octacosane. Zhang et al.13 showed that the heterogeneous reaction of OH with cholestane (a C27H48 solid cyclic alkane with a branched aliphatic side chain) yielded few ring-opening oxidation products. This is in contrast to gas phase reaction ...
LECTURE 7 REDUCTIVE ELIMINATIONSa
... • In all oxidative additions, a pair of electrons from the metal is used to break the A−B bond in the reagent. • In the SN2 pathway, adopted for polarized A‐B substrates such as alkyl halides, the metal electron pair of LnM directly attacks the A–B σ* orbital by an in‐line attack at the least electr ...
... • In all oxidative additions, a pair of electrons from the metal is used to break the A−B bond in the reagent. • In the SN2 pathway, adopted for polarized A‐B substrates such as alkyl halides, the metal electron pair of LnM directly attacks the A–B σ* orbital by an in‐line attack at the least electr ...
Ene reaction

The ene reaction (also known as the Alder-ene reaction) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Also,many useful Lewis acid-catalyzed ene reactions have been developed which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products.