Isoindolone Formation via Intramolecular Diels
... commercial manufacture, as shown in Scheme 2. The reaction was performed successfully at 100 L scale to produce approximately 14 kg of amidoxime 5 in total. To utilise this amidoxime fragment, it was important then to find a suitable commercial synthesis of the isoindolone fragment 2. The original me ...
... commercial manufacture, as shown in Scheme 2. The reaction was performed successfully at 100 L scale to produce approximately 14 kg of amidoxime 5 in total. To utilise this amidoxime fragment, it was important then to find a suitable commercial synthesis of the isoindolone fragment 2. The original me ...
Alcohols
... Alcohols, Phenols, and Ethers Alcohols, Phenols, and Ethers • Organic derivatives of water in which one or both of the water hydrogens is replaced by an organic group: H-O-H versus RO-H, Ar-O-H, and R-O-R’ Thiols and sulfides • Corresponding sulfur analogs, R-S-H and R-S-R’ The names alcohol and th ...
... Alcohols, Phenols, and Ethers Alcohols, Phenols, and Ethers • Organic derivatives of water in which one or both of the water hydrogens is replaced by an organic group: H-O-H versus RO-H, Ar-O-H, and R-O-R’ Thiols and sulfides • Corresponding sulfur analogs, R-S-H and R-S-R’ The names alcohol and th ...
Organic Synthesis II
... Mechanisms for many oxidation reactions (even well-known ones) are significantly more complex than drawn throughout this course (and in many cases are not known or understood). Some are based on factual mechanistic data; some should be treated more as a mnemonic than explanation. ...
... Mechanisms for many oxidation reactions (even well-known ones) are significantly more complex than drawn throughout this course (and in many cases are not known or understood). Some are based on factual mechanistic data; some should be treated more as a mnemonic than explanation. ...
52 - University of Strathclyde
... important breakthroughs in transition-metal catalysis.12 Furthermore, recent reports have shown that the use of NHC's in a stabilising role has been crucial for the isolation of highly reactive molecules containing low oxidation state main-group elements.13 Surprisingly, despite their important appl ...
... important breakthroughs in transition-metal catalysis.12 Furthermore, recent reports have shown that the use of NHC's in a stabilising role has been crucial for the isolation of highly reactive molecules containing low oxidation state main-group elements.13 Surprisingly, despite their important appl ...
Full-Text PDF
... for solid-phase peptide synthesis (SPPS) [15–19]. Special attention has been devoted to the development of protecting groups and linkers, which are the cornerstones of SPPS. Most of these are based on the benzyl (Bzl) moiety. To make this moiety more acid-labile and therefore more user friendly, we ...
... for solid-phase peptide synthesis (SPPS) [15–19]. Special attention has been devoted to the development of protecting groups and linkers, which are the cornerstones of SPPS. Most of these are based on the benzyl (Bzl) moiety. To make this moiety more acid-labile and therefore more user friendly, we ...
File
... HO OH HO OH equivalent). CF3 is a strong EWG, so O H CH3 H CF3 destabilizes the δ+ of the carbonyl, H CH3 G G+H-hydrate making the CF3 carbonyl higher in energy than the aldehyde. This makes the hydrate reaction of the CF3 carbonyl downhill, resulting in a higher amount of hydrate. 16. In each pair, ...
... HO OH HO OH equivalent). CF3 is a strong EWG, so O H CH3 H CF3 destabilizes the δ+ of the carbonyl, H CH3 G G+H-hydrate making the CF3 carbonyl higher in energy than the aldehyde. This makes the hydrate reaction of the CF3 carbonyl downhill, resulting in a higher amount of hydrate. 16. In each pair, ...
Chapter 12
... amine adduct with BF3 . Since the enthalpy of adduct formation is least favorable with BF3, however, it is concluded that the loss in BX double-bond character upon rehybridization to form an adduct is greater with BF3 than in the other tri halides. From this we can conclude that the double-bond cha ...
... amine adduct with BF3 . Since the enthalpy of adduct formation is least favorable with BF3, however, it is concluded that the loss in BX double-bond character upon rehybridization to form an adduct is greater with BF3 than in the other tri halides. From this we can conclude that the double-bond cha ...
Synthesis of a TREN in Which the Aryl Substituents are... Atom Macrocycle ̈ller *
... recrystallization and was fully characterized by 1H and 13C{1H} NMR spectroscopy. C2-Symmetric 8b-2/1 is the result of two “interarm” metathesis reactions and one “intraarm” metathesis reaction. It is not known whether two “interarm” metathesis reactions precede the one “intraarm” metathesis reactio ...
... recrystallization and was fully characterized by 1H and 13C{1H} NMR spectroscopy. C2-Symmetric 8b-2/1 is the result of two “interarm” metathesis reactions and one “intraarm” metathesis reaction. It is not known whether two “interarm” metathesis reactions precede the one “intraarm” metathesis reactio ...
Q4) How the following conversions can be carried out?
... Q4. How do you account for the fact that unlike phenol, 2, 4-dinitrophenol and 2, 4, 6-trinitrophenol are soluble in aqueous solution of sodium carbonate? ANS 2, 4-Dinitrophenol and 2, 4, 6-trinitrophenol are stronger acids then carbonic acid (H2CO3) due to the presence of electron withdrawing – NO ...
... Q4. How do you account for the fact that unlike phenol, 2, 4-dinitrophenol and 2, 4, 6-trinitrophenol are soluble in aqueous solution of sodium carbonate? ANS 2, 4-Dinitrophenol and 2, 4, 6-trinitrophenol are stronger acids then carbonic acid (H2CO3) due to the presence of electron withdrawing – NO ...
Lokshin2011
... 2. When irradiated by a mercury lamp, the CO molecule is abstracted from tricarbonyl complexes and dicarbonyl chelates are formed, stabilized by intramolecular coordination of the manganese atom with a substituent in the Cp-ring. This changes the color of the solution. In a closed system the CO mole ...
... 2. When irradiated by a mercury lamp, the CO molecule is abstracted from tricarbonyl complexes and dicarbonyl chelates are formed, stabilized by intramolecular coordination of the manganese atom with a substituent in the Cp-ring. This changes the color of the solution. In a closed system the CO mole ...
New Exp8
... Limitations of E1 Reaction: Acid-Catalyzed Dehydrations Competition can occur with SN1 reaction if reaction conditions are not ‘controlled’ (when protic solvents, non-basic nucleophiles are used). Mixtures of products form with the E1 reaction (also SN1). Unsymmetrical reagents and rearrangements po ...
... Limitations of E1 Reaction: Acid-Catalyzed Dehydrations Competition can occur with SN1 reaction if reaction conditions are not ‘controlled’ (when protic solvents, non-basic nucleophiles are used). Mixtures of products form with the E1 reaction (also SN1). Unsymmetrical reagents and rearrangements po ...
New insights into the mechanism of sorbitol transformation
... pressure separator cooled by water circulation. The liquid effluents were collected in vials at the exit of the separator to be further analyzed. After the separator, the gaseous effluent flowed through a back pressure regulator and then through a gas bulb. A drumtype gas meter measured the gas flo ...
... pressure separator cooled by water circulation. The liquid effluents were collected in vials at the exit of the separator to be further analyzed. After the separator, the gaseous effluent flowed through a back pressure regulator and then through a gas bulb. A drumtype gas meter measured the gas flo ...
Homogeneous Catalysis
... formed. Because only one reagent is involved in the rate-limiting step, the overall rate of reaction is proportional to the concentration of only this reagent. Rate = k((CH3)3CBr) The rate law for this reaction therefore differs from what we would predict from the stoichiometry of the reaction. Alth ...
... formed. Because only one reagent is involved in the rate-limiting step, the overall rate of reaction is proportional to the concentration of only this reagent. Rate = k((CH3)3CBr) The rate law for this reaction therefore differs from what we would predict from the stoichiometry of the reaction. Alth ...
Synthesis of Novel Steroid-Peptoid Hybrid Macrocycles by
... As depicted in Scheme 4 (top), an unidirectional Ugi-MiB approach can be implemented easily by utilizing the lithocholic acid derivative 17 functionalized with an amino group at C-3. This compound was readily prepared from methyl lithocholate according to reported procedures [13,24]. The 3α-OH is re ...
... As depicted in Scheme 4 (top), an unidirectional Ugi-MiB approach can be implemented easily by utilizing the lithocholic acid derivative 17 functionalized with an amino group at C-3. This compound was readily prepared from methyl lithocholate according to reported procedures [13,24]. The 3α-OH is re ...
7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic
... In fact, when R3 C-Br has fewer than two CH3 groups, it does not react at all by the S N1 mechanism (see Figure7.13). These changes in SN1 rates result from the effect of alkyl groups such as CH3 on the stability of R3 C+ that forms in the first step of the SN1 mechanism. Carbocation Stability. The ...
... In fact, when R3 C-Br has fewer than two CH3 groups, it does not react at all by the S N1 mechanism (see Figure7.13). These changes in SN1 rates result from the effect of alkyl groups such as CH3 on the stability of R3 C+ that forms in the first step of the SN1 mechanism. Carbocation Stability. The ...
Rhenium Nanochemistry for Catalyst Preparation
... a narrow pore-size distribution centered at 3 nm in the metathesis of internal as well as terminal olefins without functional groups has shown higher catalytic activity than Re2O7 on common γ–Al2O3 in liquid phase [21,22]. Rhenium oxide supported on ordered mesoporous alumina has even been claimed a ...
... a narrow pore-size distribution centered at 3 nm in the metathesis of internal as well as terminal olefins without functional groups has shown higher catalytic activity than Re2O7 on common γ–Al2O3 in liquid phase [21,22]. Rhenium oxide supported on ordered mesoporous alumina has even been claimed a ...
MS PowerPoint - Catalysis Eprints database
... CH3(CH2)7-CH=CH2-------------→CH3(CH2)7-CH2- CH2 –CHO Co(Co)6 ↓ H2 CH3(CH2)7-CH2- CH2 –CH2- OH Step.8 – Repeat of steps 5,6 & 7 to exhaustion ...
... CH3(CH2)7-CH=CH2-------------→CH3(CH2)7-CH2- CH2 –CHO Co(Co)6 ↓ H2 CH3(CH2)7-CH2- CH2 –CH2- OH Step.8 – Repeat of steps 5,6 & 7 to exhaustion ...
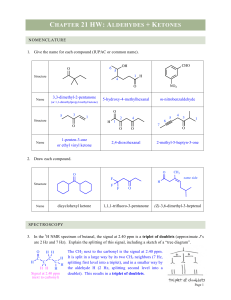
aldehyde group - Imperial Valley College Faculty Websites
... The properties of aldehydes and ketones are largely determined by the polarized carbonyl group( ...
... The properties of aldehydes and ketones are largely determined by the polarized carbonyl group( ...
Preparation of d, l-Phenylalanine by Amidocarbonylation of Benzyl
... acids is Wakamatsu's amidocarbonylation reaction3,4 which allows the preparation of amino acids from aldehydes containing at least one R-proton, CO, and a limited class of amides, in particular acetamide. The necessity of the R-proton is best explained by assuming the intermediacy of the enamide (Sc ...
... acids is Wakamatsu's amidocarbonylation reaction3,4 which allows the preparation of amino acids from aldehydes containing at least one R-proton, CO, and a limited class of amides, in particular acetamide. The necessity of the R-proton is best explained by assuming the intermediacy of the enamide (Sc ...
Organic Chemistry II Introduction
... Reaction of a phenol with strong oxidizing agents yields a quinone Fremy's salt [(KSO3)2NO] works under mild conditions through a radical mechanism ...
... Reaction of a phenol with strong oxidizing agents yields a quinone Fremy's salt [(KSO3)2NO] works under mild conditions through a radical mechanism ...
Carbohydrates: Occurrence, Structures and Chemistry
... was purely arbitrary, yet proved to be a fortunate one, since much later, in 1951, it was proven by special X-ray structural analysis [13] that he had made the right choice. The D-aldose family tree is shown in Figure 3, comprising five of the most important ...
... was purely arbitrary, yet proved to be a fortunate one, since much later, in 1951, it was proven by special X-ray structural analysis [13] that he had made the right choice. The D-aldose family tree is shown in Figure 3, comprising five of the most important ...
The bite angle makes the catalyst
... of the allyl moiety. In the transition state, the hybridisation of this carbon atom changes from sp2 to sp3, which results in a bending of the propyl group towards the phosphine. This causes steric interference of the propyl group with the diphosphine ligand. The positive effect of large bite angles ...
... of the allyl moiety. In the transition state, the hybridisation of this carbon atom changes from sp2 to sp3, which results in a bending of the propyl group towards the phosphine. This causes steric interference of the propyl group with the diphosphine ligand. The positive effect of large bite angles ...
Unsaturated hydrocarbons Alkenes
... 3-Chloro-4-ethylcyclobutene (not 1-Chloro-2-ethylcyclobutene) ...
... 3-Chloro-4-ethylcyclobutene (not 1-Chloro-2-ethylcyclobutene) ...
15: Carbonyl Compounds: Esters, Amides, and Related Molecules
... "-ic acid" of the acid name and replace it with the ending -ate. Systematic or Common. It is important to remember not to mix systematic and common nomenclature together in the name of an organic compound. For example, neither the name isoamyl butanoate or the name 2-methylpropyl butyrate are correc ...
... "-ic acid" of the acid name and replace it with the ending -ate. Systematic or Common. It is important to remember not to mix systematic and common nomenclature together in the name of an organic compound. For example, neither the name isoamyl butanoate or the name 2-methylpropyl butyrate are correc ...
13. amines - WordPress.com
... So the electron pairs are less available for protonation and hence aryl amines are less basic. Also the anilinium ion formed by accepting a proton can have only two resonating structures as follows: ...
... So the electron pairs are less available for protonation and hence aryl amines are less basic. Also the anilinium ion formed by accepting a proton can have only two resonating structures as follows: ...
Ene reaction

The ene reaction (also known as the Alder-ene reaction) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Also,many useful Lewis acid-catalyzed ene reactions have been developed which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products.