Kinetic Control of Aqueous Polymerization Using Radicals
... from the cage releasing two free radicals to the reaction medium. Radical pairs in the S state have extremely high probability to undergo recombination reactions and/or formation of cage products. Conversely, radical pairs in any of the three T states cannot recombine. Nevertheless, radicals may pas ...
... from the cage releasing two free radicals to the reaction medium. Radical pairs in the S state have extremely high probability to undergo recombination reactions and/or formation of cage products. Conversely, radical pairs in any of the three T states cannot recombine. Nevertheless, radicals may pas ...
A convenient method for the preparation of oxazaborolidine catalyst
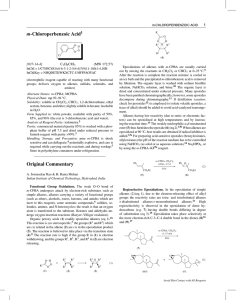
... The synthesis of enantiomerically pure compounds has become an important area of research for pharmaceutical industries, as often two enantiomers of a chiral drug molecule display different biological activities.1 Asymmetric reduction of prochiral ketones is an extremely important methodology for the ...
... The synthesis of enantiomerically pure compounds has become an important area of research for pharmaceutical industries, as often two enantiomers of a chiral drug molecule display different biological activities.1 Asymmetric reduction of prochiral ketones is an extremely important methodology for the ...
Enthalpy Barriers for Asymmetric SN2 Alkyl
... are in good agreement with those calculated using G3(MP2) theory and are on average lower by 2.5 ( 3.4 kJ mol-1. More importantly, these values are also in good agreement with those experimental values determined by us15,16 being lower on average by 3.7 ( 2.2 kJ mol-1. This lends confidence to the p ...
... are in good agreement with those calculated using G3(MP2) theory and are on average lower by 2.5 ( 3.4 kJ mol-1. More importantly, these values are also in good agreement with those experimental values determined by us15,16 being lower on average by 3.7 ( 2.2 kJ mol-1. This lends confidence to the p ...
The catalytic function of a silica gel-immobilized Mn(II)
... in the presence of aqueous sodium hydrogencarbonate. The effects of reaction parameters such as solvent, NaHCO3 and oxidant in the epoxidation of cis-cyclooctene were investigated. Cycloalkenes were oxidized efficiently to their corresponding epoxide with 87–100% selectivity in the presence of this c ...
... in the presence of aqueous sodium hydrogencarbonate. The effects of reaction parameters such as solvent, NaHCO3 and oxidant in the epoxidation of cis-cyclooctene were investigated. Cycloalkenes were oxidized efficiently to their corresponding epoxide with 87–100% selectivity in the presence of this c ...
HIGHLY SELECTIVE RHODIUM–CATALYZED C–H BORYLATIONS IN
... First and foremost I am greatly indebted to my supervisor Dr. Cathleen Crudden. The time spent working with you helped me to better understand the trade while growing both as a scientist and a person. Leaving the lab, I feel my training and experiences have best prepared me for any future challenges ...
... First and foremost I am greatly indebted to my supervisor Dr. Cathleen Crudden. The time spent working with you helped me to better understand the trade while growing both as a scientist and a person. Leaving the lab, I feel my training and experiences have best prepared me for any future challenges ...
the suzuki-miyaura reaction and boron reagents – mechanism
... Hydroboration of alkenes/alkynes. ¤ Direct hydroboration of alkynes, first descriped by Brown21,22, requires harsh reaction conditions and is functional group sensitive. ¤ Nöth reported the first rhodium catalysed selective addition of catecholborane to alkenes23. ...
... Hydroboration of alkenes/alkynes. ¤ Direct hydroboration of alkynes, first descriped by Brown21,22, requires harsh reaction conditions and is functional group sensitive. ¤ Nöth reported the first rhodium catalysed selective addition of catecholborane to alkenes23. ...
Development of Catalytic Ester Condensations and Hydrolysis of
... Since a variety of esters are synthesized on an industrial scale all over the world, large amounts of byproducts are also generated during synthetic processes. Therefore, the use of stoichiometric dehydrating reagents, activated carboxylic acid derivatives, excess amounts of substrates, or solvents ...
... Since a variety of esters are synthesized on an industrial scale all over the world, large amounts of byproducts are also generated during synthetic processes. Therefore, the use of stoichiometric dehydrating reagents, activated carboxylic acid derivatives, excess amounts of substrates, or solvents ...
Organic Chemistry
... • if DG‡ is small, many collisions occur with sufficient energy to reach the transition state; reaction is fast ...
... • if DG‡ is small, many collisions occur with sufficient energy to reach the transition state; reaction is fast ...
as a PDF
... by Lurgi (12) is shown in Figure 6. The process involved handling the circulation of fatty alcohol/ catalyst slurry phase. The CuCr catalyst consumption is rather high up to 5 kg/ton of fatty alcohol produced partly because reaction of fatty acid with copper in the catalyst. Aldehyde, alkane, wax es ...
... by Lurgi (12) is shown in Figure 6. The process involved handling the circulation of fatty alcohol/ catalyst slurry phase. The CuCr catalyst consumption is rather high up to 5 kg/ton of fatty alcohol produced partly because reaction of fatty acid with copper in the catalyst. Aldehyde, alkane, wax es ...
ether - HCC Southeast Commons
... Practice Problem: Why do you suppose only symmetrical ethers are prepared by the sulfuric acid-catalyzed dehydration procedure? What product(s) would you expect if ethanol and 1-propanol were allowed to react together? In what ratio would the products be formed if the two alcohols were of equal rea ...
... Practice Problem: Why do you suppose only symmetrical ethers are prepared by the sulfuric acid-catalyzed dehydration procedure? What product(s) would you expect if ethanol and 1-propanol were allowed to react together? In what ratio would the products be formed if the two alcohols were of equal rea ...
Chemistry of Fatty Acids
... reactivity. Only rarely do saturated chains show reactivity. Carboxyl groups and unsaturated centers usually react independently, but when in close proximity, both may react through neighboring group participation. In enzymatic reactions, the reactivity of the carboxyl group can be influenced by the ...
... reactivity. Only rarely do saturated chains show reactivity. Carboxyl groups and unsaturated centers usually react independently, but when in close proximity, both may react through neighboring group participation. In enzymatic reactions, the reactivity of the carboxyl group can be influenced by the ...
Chloroperbenzoic_aci..
... an ice bath and the precipitated m-chlorobenzoic acid is removed by filtration. The organic layer is washed with sodium bisulfite solution, NaHCO3 solution, and brine.10 The organic layer is dried and concentrated under reduced pressure. Many epoxides have been purified chromatographically; however, ...
... an ice bath and the precipitated m-chlorobenzoic acid is removed by filtration. The organic layer is washed with sodium bisulfite solution, NaHCO3 solution, and brine.10 The organic layer is dried and concentrated under reduced pressure. Many epoxides have been purified chromatographically; however, ...
aldehydes and ketones
... Lower members of aldehydes and ketones ( upto C4) are soluble in water due to H-bonding between polar carbonyl group and water. However, solubility decreases with increase in molecular weight Aromatic aldehydes and ketones are much less soluble than corresponding aliphatic aldehydes and ketone ...
... Lower members of aldehydes and ketones ( upto C4) are soluble in water due to H-bonding between polar carbonyl group and water. However, solubility decreases with increase in molecular weight Aromatic aldehydes and ketones are much less soluble than corresponding aliphatic aldehydes and ketone ...
chemical kinetics
... Bimolecular reactions involve simultaneous collision between two species, for example, dissociation of hydrogen iodide. 2HI → H2 + I2 Trimolecular or termolecular reactions involve simultaneous collision between three reacting species, for example, 2NO + O2 → 2NO2 The probability that more than thre ...
... Bimolecular reactions involve simultaneous collision between two species, for example, dissociation of hydrogen iodide. 2HI → H2 + I2 Trimolecular or termolecular reactions involve simultaneous collision between three reacting species, for example, 2NO + O2 → 2NO2 The probability that more than thre ...
Chpt 23Final7e
... (a) How do you account for the fact that the -NH3 + group of the conjugate acid of alanine is a stronger acid than the -NH3 + group of the conjugate acid of isopropylamine? The electron-withdrawing properties of the carboxyl group adjacent to the amine of alanine make the conjugate acid of alanine m ...
... (a) How do you account for the fact that the -NH3 + group of the conjugate acid of alanine is a stronger acid than the -NH3 + group of the conjugate acid of isopropylamine? The electron-withdrawing properties of the carboxyl group adjacent to the amine of alanine make the conjugate acid of alanine m ...
Improved Synthesis, Separation, Transition Metal Coordination and
... Gel permeation chromatography of the white solid produced from three reactions of 1-hexene, 1-octene, and a mixture of 1- hexene/1octene and Ni2Cl4(meso-et,ph-P4) in a H2O/acetone solvent mixture (70°C) .............................................................................................. 26 ...
... Gel permeation chromatography of the white solid produced from three reactions of 1-hexene, 1-octene, and a mixture of 1- hexene/1octene and Ni2Cl4(meso-et,ph-P4) in a H2O/acetone solvent mixture (70°C) .............................................................................................. 26 ...
CH 2
... Alkenes are relatively stable compounds, but are more reactive than alkanes. This is compatible with the idea that the carbon-carbon double bond in alkenes is stronger than the carbon-carbon single bond in alkanes, however, as the majority of the reactions of alkenes involve the rupture of this bo ...
... Alkenes are relatively stable compounds, but are more reactive than alkanes. This is compatible with the idea that the carbon-carbon double bond in alkenes is stronger than the carbon-carbon single bond in alkanes, however, as the majority of the reactions of alkenes involve the rupture of this bo ...
Unit 1 - Physical Chemistry REACTION KINETICS
... The rate determing step would be step ① as it is the slow step and only chemicals involved in the rate determining step would effect the rate of reaction. HI is a reaction intermediate and would be used up as fast as it is produced. Proving a mechanism can be very difficult. Detecting intermediates ...
... The rate determing step would be step ① as it is the slow step and only chemicals involved in the rate determining step would effect the rate of reaction. HI is a reaction intermediate and would be used up as fast as it is produced. Proving a mechanism can be very difficult. Detecting intermediates ...
Organic Chemistry
... Esters are RC(=O)-Z compounds where Z is OR', and R' groups are alkyl or aryl. Preparation, Properties, and Reactivity (15.3A) The differences between esters and acid halides are much greater then their similarities. Preparation. We previously described the preparation of esters from acid halides an ...
... Esters are RC(=O)-Z compounds where Z is OR', and R' groups are alkyl or aryl. Preparation, Properties, and Reactivity (15.3A) The differences between esters and acid halides are much greater then their similarities. Preparation. We previously described the preparation of esters from acid halides an ...
15: Carbonyl Compounds: Esters, Amides, and Related Molecules
... need to be the same as each other and can be any mixture of H, alkyl, or aryl groups (Figure 15.33). Figure 15.33 We find the amide functional group in many naturally occuring compounds. For example, we will see below, and also in Chapter 22, that it is the repeating unit in the backbone of all prot ...
... need to be the same as each other and can be any mixture of H, alkyl, or aryl groups (Figure 15.33). Figure 15.33 We find the amide functional group in many naturally occuring compounds. For example, we will see below, and also in Chapter 22, that it is the repeating unit in the backbone of all prot ...
ALCOHOLS AND ETHERS
... expected to be similar to those of water, H O H , the simplest hydroxylic compound. Alcohols, ROH, can be regarded in this respect as substitution products of water. However, with alcohols we shall be interested not only in reactions that proceed at the 8-H bond but also with processes that result i ...
... expected to be similar to those of water, H O H , the simplest hydroxylic compound. Alcohols, ROH, can be regarded in this respect as substitution products of water. However, with alcohols we shall be interested not only in reactions that proceed at the 8-H bond but also with processes that result i ...
A >200 meV Uphill Thermodynamic Landscape for Radical
... Y122• reversibly oxidizes C439 via multiple proton-coupled electron transfer (PCET) steps through a pathway involving aromatic amino acid residues Y122 ⇆ [W48] ⇆ Y356 in β2 to Y731 ⇆ Y730 ⇆ C439 in α2. Currently, there is no direct evidence for the involvement of W48 in RT.9−11 In the wild-type (wt) ...
... Y122• reversibly oxidizes C439 via multiple proton-coupled electron transfer (PCET) steps through a pathway involving aromatic amino acid residues Y122 ⇆ [W48] ⇆ Y356 in β2 to Y731 ⇆ Y730 ⇆ C439 in α2. Currently, there is no direct evidence for the involvement of W48 in RT.9−11 In the wild-type (wt) ...
Formation Mechanisms of Naphthalene and
... naphthalene after consecutive dehydrogenation steps. Alternatively, the reactions involving PES with x = 6 and 7 can lead to naphthalene via hydrogen atom additions if hydrogen atoms are available. One of the reactants usually contains an aromatic six-member ring so that the reaction can proceed as ...
... naphthalene after consecutive dehydrogenation steps. Alternatively, the reactions involving PES with x = 6 and 7 can lead to naphthalene via hydrogen atom additions if hydrogen atoms are available. One of the reactants usually contains an aromatic six-member ring so that the reaction can proceed as ...
Ene reaction

The ene reaction (also known as the Alder-ene reaction) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Also,many useful Lewis acid-catalyzed ene reactions have been developed which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products.