dr.ebtehal Lec3
... • Drug metabolism can occur in every tissue (e.g. gut, lung and kidney). However, the major drug metabolizing enzymes (DMEs) are expressed at the highest levels in the liver, which thus serves as the major organ of metabolic clearance • Drug metabolism serves to control the exposure of a potentially ...
... • Drug metabolism can occur in every tissue (e.g. gut, lung and kidney). However, the major drug metabolizing enzymes (DMEs) are expressed at the highest levels in the liver, which thus serves as the major organ of metabolic clearance • Drug metabolism serves to control the exposure of a potentially ...
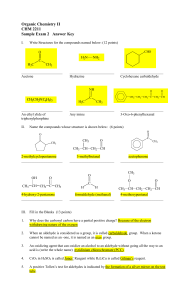
Organic Chemistry II CHM 2211 Sample Exam 2 Answer Key
... Why does the carbonyl carbon have a partial positive charge? Because of the electron withdrawing nature of the oxygen ...
... Why does the carbonyl carbon have a partial positive charge? Because of the electron withdrawing nature of the oxygen ...
Catalytic Asymmetric Induction. Highly Enantioselective Addition of
... took place smoothly, as under the above described catalytic conditions, leading to the desired alcohol in 98% ee and in 88% (benza1dehyde:diethyIzinc:DAIB = 1:2:1, 12 h) or 49% (1:1:0.5 ratio, 12 h) yield. These results indicate that two zinc species per aldehyde are responsible for the alkylation r ...
... took place smoothly, as under the above described catalytic conditions, leading to the desired alcohol in 98% ee and in 88% (benza1dehyde:diethyIzinc:DAIB = 1:2:1, 12 h) or 49% (1:1:0.5 ratio, 12 h) yield. These results indicate that two zinc species per aldehyde are responsible for the alkylation r ...
Transition Metal Catalyzed Selective Oxidation of Sugars and Polyols
... the reaction of the 16-electron complex with a hydrogen donor and that a kinetic model must account for two effectively different oxidation and reduction catalysts. In an alcohol/ketone equal-concentration experiment with Noyori-type ruthenium(II) complexes a linear relationship was found between th ...
... the reaction of the 16-electron complex with a hydrogen donor and that a kinetic model must account for two effectively different oxidation and reduction catalysts. In an alcohol/ketone equal-concentration experiment with Noyori-type ruthenium(II) complexes a linear relationship was found between th ...
Transition Metal Reagents and Catalysts
... ®rst by giving a simple mechanistic explanation in chapter 2. Then a number of important types of reactions classi®ed mainly by representative substrates such as organic halides and allylic derivatives are surveyed with pertinent examples. For this purpose, I cited many references; these were select ...
... ®rst by giving a simple mechanistic explanation in chapter 2. Then a number of important types of reactions classi®ed mainly by representative substrates such as organic halides and allylic derivatives are surveyed with pertinent examples. For this purpose, I cited many references; these were select ...
Chem E2b - Organic Chemistry II What is Organic Chemistry?
... Lewis Acids and Bases • Lewis acid: non-proton-donating acid; will accept two electrons • Lewis base: electron pair donors ...
... Lewis Acids and Bases • Lewis acid: non-proton-donating acid; will accept two electrons • Lewis base: electron pair donors ...
Curriculum Vitae
... 106. Deluca, R. J.; Stokes, B. J.; Sigman, M. S. “The strategic generation and interception of palladiumhydrides for use in alkene functionalization reactions,” Pure Appl. Chem. 2014, 86, 395-408. 105. Xu, L.; Hilton, M. J.; Zhang, X.; Norrby, P.-O.*; Wu, Y.-D.*; Sigman, M. S.*; Wiest, O.* “Mechanis ...
... 106. Deluca, R. J.; Stokes, B. J.; Sigman, M. S. “The strategic generation and interception of palladiumhydrides for use in alkene functionalization reactions,” Pure Appl. Chem. 2014, 86, 395-408. 105. Xu, L.; Hilton, M. J.; Zhang, X.; Norrby, P.-O.*; Wu, Y.-D.*; Sigman, M. S.*; Wiest, O.* “Mechanis ...
The Grob Fragmentation
... - Double bonds are generated in the fragmentation - It has been shown to work for usually hard to generate double bonds (Baron example) - Generally if all the stereochemical requirements are met for a concerted mechanism (i.e. an anti-peri planar relationship) side reactions can be suppressed and yo ...
... - Double bonds are generated in the fragmentation - It has been shown to work for usually hard to generate double bonds (Baron example) - Generally if all the stereochemical requirements are met for a concerted mechanism (i.e. an anti-peri planar relationship) side reactions can be suppressed and yo ...
CH221 CLASS 13
... In practice, aqueous dimethyl sulfoxide (DMSO) is often used, in order to solubilize the alkene, and N-bromosuccinimide (NBS) is used as a (safer) supply of bromine. E.g. O ...
... In practice, aqueous dimethyl sulfoxide (DMSO) is often used, in order to solubilize the alkene, and N-bromosuccinimide (NBS) is used as a (safer) supply of bromine. E.g. O ...
Lithium Bromide Original Commentary
... acid in a variety of reactions. For example, this reagent was used in the Pictet-Spengler cyclization of a highly functionalized imine (eq 13).33 In this reaction, carbon-carbon bond formation occurs without reaction or loss of stereochemical integrity of the α-amino nitrile functionality. Lithium b ...
... acid in a variety of reactions. For example, this reagent was used in the Pictet-Spengler cyclization of a highly functionalized imine (eq 13).33 In this reaction, carbon-carbon bond formation occurs without reaction or loss of stereochemical integrity of the α-amino nitrile functionality. Lithium b ...
Diastereoselective Allylation of Carbonyl Compounds and Imines:
... units in almost enantiomerically pure form.10 The reactions were performed in dichloromethane at −78 °C. Importantly, the stoichiometry of the Lewis acid determined the relative configuration of the three stereogenic centers. Thus, when 1 equiv of SnCl4 was used syn−anti triols were obtained (Table 1 ...
... units in almost enantiomerically pure form.10 The reactions were performed in dichloromethane at −78 °C. Importantly, the stoichiometry of the Lewis acid determined the relative configuration of the three stereogenic centers. Thus, when 1 equiv of SnCl4 was used syn−anti triols were obtained (Table 1 ...
Elimination Reactions
... • This tells us that the C-D or C-H bonds are not broken in the rate determining step (step 1). They are broken in the fast step (step 2) in the mechanism). ...
... • This tells us that the C-D or C-H bonds are not broken in the rate determining step (step 1). They are broken in the fast step (step 2) in the mechanism). ...
R - Evans - Harvard University
... the amino acid R group, as it is in the alkylation route, thereby affording access to classes of targets, e.g., arylglycines and tert-alkylglycines, that are unavailable by other enolate-based methodology. Finally, precedent would indicate that any stereochemical defect in the amination process coul ...
... the amino acid R group, as it is in the alkylation route, thereby affording access to classes of targets, e.g., arylglycines and tert-alkylglycines, that are unavailable by other enolate-based methodology. Finally, precedent would indicate that any stereochemical defect in the amination process coul ...
Iodomethylzinc_iodid.. - Groupe Charette
... the Zn–Cu couple with CH2 I2 and a crystal of Iodine in Et2 O followed by heating to reflux generates the active reagent. Other modifications include the use of CH2 I2 /Zn/CuCl,12a CH2 I2 /Zn–Ag couple,12b CH2 Br2 /Zn/TiCl4 ,12c and CH2 Br2 /Zn/AcCl/CuCl.12d Type 2 reagent generation has been utiliz ...
... the Zn–Cu couple with CH2 I2 and a crystal of Iodine in Et2 O followed by heating to reflux generates the active reagent. Other modifications include the use of CH2 I2 /Zn/CuCl,12a CH2 I2 /Zn–Ag couple,12b CH2 Br2 /Zn/TiCl4 ,12c and CH2 Br2 /Zn/AcCl/CuCl.12d Type 2 reagent generation has been utiliz ...
organic practice problems
... e. CHFCF2 ____ 79. All of the following statements concerning polymers are correct EXCEPT a. elastomers are materials that spring back to their original shape when stretched. b. polymers formed from two or more different monomers are called copolymers. c. thermoplastics can withstand very high tempe ...
... e. CHFCF2 ____ 79. All of the following statements concerning polymers are correct EXCEPT a. elastomers are materials that spring back to their original shape when stretched. b. polymers formed from two or more different monomers are called copolymers. c. thermoplastics can withstand very high tempe ...
Carbonyl Condensation Reactions
... carbonyl compound becomes the nucleophilic enolate and which reacts at the electrophilic carbonyl carbon. The strategy of a directed aldol reaction is as follows: [1] Prepare the enolate of one carbonyl component with LDA. [2] Add the second carbonyl compound (the electrophile) to this enolate. Beca ...
... carbonyl compound becomes the nucleophilic enolate and which reacts at the electrophilic carbonyl carbon. The strategy of a directed aldol reaction is as follows: [1] Prepare the enolate of one carbonyl component with LDA. [2] Add the second carbonyl compound (the electrophile) to this enolate. Beca ...
hydrogen peroxide disproportionation and organic
... thank Dr. Ana Ison for all of her support during this process. Ana was always around to discuss ideas about projects. I would also like thank her for the help she provided in acquiring the GC data. I would especially like to thank Dr. Celeste Regino. Celeste taught me the intricacies of HPLC and tha ...
... thank Dr. Ana Ison for all of her support during this process. Ana was always around to discuss ideas about projects. I would also like thank her for the help she provided in acquiring the GC data. I would especially like to thank Dr. Celeste Regino. Celeste taught me the intricacies of HPLC and tha ...
Reduction of CuO in H2: in situ time
... mass spectrometer. This experiment was repeated many times and no diffraction lines for Cu4 O3 [16] or Cu2 O [17] were seen during the reduction. We also investigated the reduction process at lower temperatures, as shown in figure 2. The decrease in reaction temperature led to an increase in the magni ...
... mass spectrometer. This experiment was repeated many times and no diffraction lines for Cu4 O3 [16] or Cu2 O [17] were seen during the reduction. We also investigated the reduction process at lower temperatures, as shown in figure 2. The decrease in reaction temperature led to an increase in the magni ...
On The catalytic Hydrogenation of Co2 and Carboxylic acid esters
... transformation, a new concept of formate-based hydrogen battery was put forward and realized practically on a lab-scale. Beller and co-workers reported on the possibility of a cyclic storage of H2 by CO2 hydrogenation and subsequent H2 release by HCOOH decomposition in a catalytic system comprised o ...
... transformation, a new concept of formate-based hydrogen battery was put forward and realized practically on a lab-scale. Beller and co-workers reported on the possibility of a cyclic storage of H2 by CO2 hydrogenation and subsequent H2 release by HCOOH decomposition in a catalytic system comprised o ...
Ch. 6 - Department of Chemistry and Biochemistry
... The reaction coordinate indicates the progress of the reaction, in terms of the conversion of reactants to products The top of the energy curve corresponds to the transition state for the reaction The free energy of activation (DG‡) for the reaction is the difference in energy between the reactants ...
... The reaction coordinate indicates the progress of the reaction, in terms of the conversion of reactants to products The top of the energy curve corresponds to the transition state for the reaction The free energy of activation (DG‡) for the reaction is the difference in energy between the reactants ...
OC 2/e Ch 15
... most important types of nucleophilic additions to a C=O group; a new carbon-carbon bond is formed in the process We study addition of these carbon nucleophiles ...
... most important types of nucleophilic additions to a C=O group; a new carbon-carbon bond is formed in the process We study addition of these carbon nucleophiles ...
Chapter - FIU Faculty Websites
... • Like gem-diol formation, the synthesis of acetals is reversible, and often, the equilibrium favors the reactants. • In acetal synthesis, since water is formed as a by-product, the equilibrium can be driven to the right by removing H2O as it is formed using distillation or other techniques. Please ...
... • Like gem-diol formation, the synthesis of acetals is reversible, and often, the equilibrium favors the reactants. • In acetal synthesis, since water is formed as a by-product, the equilibrium can be driven to the right by removing H2O as it is formed using distillation or other techniques. Please ...
For Peer Review Only
... Triglycerides with secondary hydroxyl functions were used for polyurethane synthesis 3. However, considering the higher reactivity of primary alcohols towards ...
... Triglycerides with secondary hydroxyl functions were used for polyurethane synthesis 3. However, considering the higher reactivity of primary alcohols towards ...
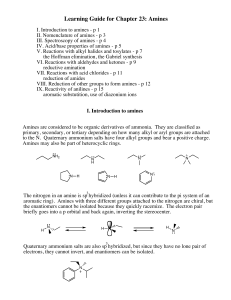
Learning Guide for Chapter 23: Amines
... Once an amine has been protonated, it is an ionic compound. Amine salts are solids, and are usually soluble in water but insoluble in organic solvents. Most biologically active amines are used as their amine salts, which dissolve to make injectable or drinkable water solutions, and are also less pro ...
... Once an amine has been protonated, it is an ionic compound. Amine salts are solids, and are usually soluble in water but insoluble in organic solvents. Most biologically active amines are used as their amine salts, which dissolve to make injectable or drinkable water solutions, and are also less pro ...
Ene reaction

The ene reaction (also known as the Alder-ene reaction) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Also,many useful Lewis acid-catalyzed ene reactions have been developed which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products.