Organic compounds
... • Carbon compounds – Organic compounds- primarily made of carbon • Carbon can from four covalent bonds • As a result, carbon can bon in a number of ways ...
... • Carbon compounds – Organic compounds- primarily made of carbon • Carbon can from four covalent bonds • As a result, carbon can bon in a number of ways ...
The structure of Matter
... O Compounds that contain only carbon and hydrogen are called hydrocarbons. O Two of the simplest hydrocarbons are methane and ethane. O Many hydrocarbons are used as fuels. ...
... O Compounds that contain only carbon and hydrogen are called hydrocarbons. O Two of the simplest hydrocarbons are methane and ethane. O Many hydrocarbons are used as fuels. ...
Hydrocarbon - TeacherWeb
... hydrogen atoms, and electrons that are shared among all six carbons in the ring (Draw the structure.) 2. Aromatic Compound: an organic compound that contains a benzene ring ...
... hydrogen atoms, and electrons that are shared among all six carbons in the ring (Draw the structure.) 2. Aromatic Compound: an organic compound that contains a benzene ring ...
Nahla abd elmoaty mohamed
... Chemical and biological studies on some Heterocyclic nitrogen derivatives ...
... Chemical and biological studies on some Heterocyclic nitrogen derivatives ...
Which of the following does not explain carbon`s ability to form long
... to form long chains. A. Carbon can form 4 covalent bonds. B. Carbon can form 4 ionic bonds. C. Carbon only has four electrons in its outer energy level. D. Carbon can bond with four other molecules. ...
... to form long chains. A. Carbon can form 4 covalent bonds. B. Carbon can form 4 ionic bonds. C. Carbon only has four electrons in its outer energy level. D. Carbon can bond with four other molecules. ...
CfE Higher Chemistry Homework Unit 2: Natures Chemistry Soaps
... c. Due to Hydrogen bonding 2-phenylethanol forms a vapour less readily that pcresyl acetate. Draw another molecule of 2-phenylethanol and use a dotted line to show where a hydrogen bond exists between the two molecules ...
... c. Due to Hydrogen bonding 2-phenylethanol forms a vapour less readily that pcresyl acetate. Draw another molecule of 2-phenylethanol and use a dotted line to show where a hydrogen bond exists between the two molecules ...
CHEMISTRY VOCABULARY
... COVALENT COMPOUNDS are formed between non metals, bonds contain shared pairs of electrons. If you know something about SALT (sodium chloride) you know something about IONIC COMPOUNDS IONIC COMPOUNDS are like salt, crystalline solids, with high melting and boiling points, they are usually soluble in ...
... COVALENT COMPOUNDS are formed between non metals, bonds contain shared pairs of electrons. If you know something about SALT (sodium chloride) you know something about IONIC COMPOUNDS IONIC COMPOUNDS are like salt, crystalline solids, with high melting and boiling points, they are usually soluble in ...
compound - Coal City Unit #1
... 1st letter is always capitalized, second letter is always sm. case • most symbols come from their names • some symbols come from Latin or Greek names • some elem. named in honor of person or place they were discovered • ea. elem. has its own unique set of chem. and physical props. ...
... 1st letter is always capitalized, second letter is always sm. case • most symbols come from their names • some symbols come from Latin or Greek names • some elem. named in honor of person or place they were discovered • ea. elem. has its own unique set of chem. and physical props. ...
Mon Feb 15 lecture
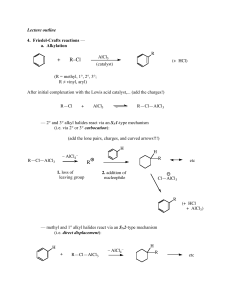
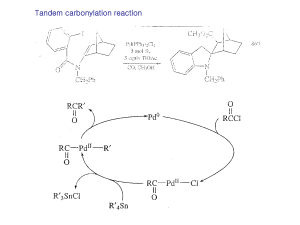
... (hydrazine, base, protic solvent, and lots of heat) avoids the rearrangement problem. (We’ll learn a bit more about these when we get to carbonyl chemistry.) — F-C acylation is quite a general procedure; a variety of different acyl groups can be introduced by this method. Here an a couple of other e ...
... (hydrazine, base, protic solvent, and lots of heat) avoids the rearrangement problem. (We’ll learn a bit more about these when we get to carbonyl chemistry.) — F-C acylation is quite a general procedure; a variety of different acyl groups can be introduced by this method. Here an a couple of other e ...
Review (Chapter 1) 1) Indicate if the following statements are true
... ) Polarity of bonds depends on their atonmic size. ) Polarity of molecules depends on their melting points ) The electronegativity increases across a period from left to right ) Structrural isomers differ in the arrangement of atoms ) constitutional isomers are structural isomers ) structural isomer ...
... ) Polarity of bonds depends on their atonmic size. ) Polarity of molecules depends on their melting points ) The electronegativity increases across a period from left to right ) Structrural isomers differ in the arrangement of atoms ) constitutional isomers are structural isomers ) structural isomer ...
슬라이드 1
... The reactivity depends on the following factors a) The number of electrons that can be accommodated by the metal orbitals b) the oxidation level of the metal, c) the electron character of other ligands on the metal ...
... The reactivity depends on the following factors a) The number of electrons that can be accommodated by the metal orbitals b) the oxidation level of the metal, c) the electron character of other ligands on the metal ...
Atomic structure and bonding I can name group 1, 7 and 0 of the
... I can state the definition of an isotope. I can state the meaning of atomic number and mass number. I can use the atomic number and mass number to determine the number of protons, neutrons and electrons within an atom. I can use the data book to write the electronic arrangement of the first 20 eleme ...
... I can state the definition of an isotope. I can state the meaning of atomic number and mass number. I can use the atomic number and mass number to determine the number of protons, neutrons and electrons within an atom. I can use the data book to write the electronic arrangement of the first 20 eleme ...
Summer Assignment 2015
... Name ________________________________ Complete this assignment on a separate sheet(s) of paper. To receive full credit you must show all work, report your answers to the proper number of significant figures, and your answers must have the appropriate units. Refer to the links on the last page for he ...
... Name ________________________________ Complete this assignment on a separate sheet(s) of paper. To receive full credit you must show all work, report your answers to the proper number of significant figures, and your answers must have the appropriate units. Refer to the links on the last page for he ...
topic-2.doc
... Electrons are in orbit around the nucleus, are involved in chemical reactions. o Orbital: three-dimensional space where an electron will most likely be found 90% of the time o First energy level: one s orbital, holds 2 electrons o Second energy level: one s and three p orbitals, holds 8 electrons Ch ...
... Electrons are in orbit around the nucleus, are involved in chemical reactions. o Orbital: three-dimensional space where an electron will most likely be found 90% of the time o First energy level: one s orbital, holds 2 electrons o Second energy level: one s and three p orbitals, holds 8 electrons Ch ...
Organic and Biochemical Compounds
... • They are called like alkanes but with the – ane ending replaced by –ene, they are different because they have at least one double covalent bond: C=C • Ex: - ethene (or ethylene) C2H4 is formed when fruit ripens -propene (or propylene) C3H6 is used to make rubbing alcohol and some plastics ...
... • They are called like alkanes but with the – ane ending replaced by –ene, they are different because they have at least one double covalent bond: C=C • Ex: - ethene (or ethylene) C2H4 is formed when fruit ripens -propene (or propylene) C3H6 is used to make rubbing alcohol and some plastics ...
Elements, Compounds, and Molecules
... All (living and nonliving) of the different kinds of matter in the universe is made from about 100 different substances, called elements. Elements are called the building blocks of matter because all matter is composed of elements. Each element is made up of the same type of atoms. ...
... All (living and nonliving) of the different kinds of matter in the universe is made from about 100 different substances, called elements. Elements are called the building blocks of matter because all matter is composed of elements. Each element is made up of the same type of atoms. ...
Chemistry Notes
... b) Neutron – Neutral particles. C. For the Elements, the number of electrons in an atom is equal to the number of Protons. This is called the Atomic Number. ...
... b) Neutron – Neutral particles. C. For the Elements, the number of electrons in an atom is equal to the number of Protons. This is called the Atomic Number. ...
Structure and Shape - Chapter 12 Valence Shell Electron Pair
... positions around the central atom so that they are as far away from each other as possible. This minimizes the potential energy of the molecule, something molecules like. ...
... positions around the central atom so that they are as far away from each other as possible. This minimizes the potential energy of the molecule, something molecules like. ...
Compounds
... A polymer is a compound made up of many smaller compounds. Some polymers include proteins, DNA, and polyethylene. For example, proteins make up your hair, your skin, and your ...
... A polymer is a compound made up of many smaller compounds. Some polymers include proteins, DNA, and polyethylene. For example, proteins make up your hair, your skin, and your ...
Chemical Formulas
... Usually transition metals have more then one oxidation number also lead and tin Check ion sheet ...
... Usually transition metals have more then one oxidation number also lead and tin Check ion sheet ...
Homoaromaticity

Homoaromaticity in organic chemistry refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.The concept of homoaromaticity was pioneered by Saul Winstein in 1959, prompted by his studies of the “tris-homocyclopropenyl” cation. Since the publication of Winstein's paper, much research has been devoted to understanding and classifying these molecules, which represent an additional “class” of aromatic molecules included under the continuously broadening definition of aromaticity. To date, homoaromatic compounds are known to exist as cationic and anionic species, and some studies support the existence of neutral homoaromatic molecules, though these are less common. The 'homotropylium' cation (C8H9+) is perhaps the best studied example of a homoaromatic compound.