Learning Guide: Water and Biomolecules (cont.) Bill Activity #17 To
... o Describe how Stanley Miller bring the abiotic synthesis of organic compounds into the context of evolution. Carbon atoms can form diverse molecules by bonding to four other atoms. o Make an electron distribution diagram of carbon. List the number of valence electrons, number of bonds and types o ...
... o Describe how Stanley Miller bring the abiotic synthesis of organic compounds into the context of evolution. Carbon atoms can form diverse molecules by bonding to four other atoms. o Make an electron distribution diagram of carbon. List the number of valence electrons, number of bonds and types o ...
(Q.3) Carbon completes its octet by
... (Q.6) The hydrocarbons having the general formula of CnH2n-2 , CnH2n+2 and CnH2n+1 are known as (Q.7) The next higher homologue of pentane and propylene is (Q.8) The IUPAC name of formaldehyde and acetaldehyde is ________and ________ (Q.9) What is meant by homologous series? State any four character ...
... (Q.6) The hydrocarbons having the general formula of CnH2n-2 , CnH2n+2 and CnH2n+1 are known as (Q.7) The next higher homologue of pentane and propylene is (Q.8) The IUPAC name of formaldehyde and acetaldehyde is ________and ________ (Q.9) What is meant by homologous series? State any four character ...
ORGANOMETALLIC COMPOUNDS
... Electron Counting in organometallic compounds: 18‐electon rule (N.V. Sigwick Rule): by applying this rule one can make predictions about the stability of complexes, which is high in case of 18 electrons (noble gas configuration) To count the electrons one has to add all valence e ...
... Electron Counting in organometallic compounds: 18‐electon rule (N.V. Sigwick Rule): by applying this rule one can make predictions about the stability of complexes, which is high in case of 18 electrons (noble gas configuration) To count the electrons one has to add all valence e ...
Introduction - INTEC Chemistry Blog
... Substituent Effects in Aromatic Rings • Substituents can cause a compound to be (much) more or (much) less reactive than benzene • Substituents affect the orientation of the reaction – the positional relationship is controlled – ortho- and para-directing activators, ortho- and para-directing deacti ...
... Substituent Effects in Aromatic Rings • Substituents can cause a compound to be (much) more or (much) less reactive than benzene • Substituents affect the orientation of the reaction – the positional relationship is controlled – ortho- and para-directing activators, ortho- and para-directing deacti ...
ORDANOCHROMIUM CHEMISTRY SUPPORTED BY -DIIMINE LIGANDS
... α-Diimine ligands can accept up to two electrons; thus they can be used to stabilize organometallic compounds in unusually low formal oxidation states of the central metal. This redox ambiguity may be useful for facilitating catalytic reactions involving different oxidation states. We are exploring ...
... α-Diimine ligands can accept up to two electrons; thus they can be used to stabilize organometallic compounds in unusually low formal oxidation states of the central metal. This redox ambiguity may be useful for facilitating catalytic reactions involving different oxidation states. We are exploring ...
directed reading a
... a. what colors the atoms are b. how the atoms are connected c. how heavy the atoms are d. what size the atoms are _____ 4. What do the backbones of some compounds have hundreds or thousands of? a. carbon atoms c. structural formulas b. carbon molecules d. acid ions HYDROCARBONS AND OTHER ORGANIC COM ...
... a. what colors the atoms are b. how the atoms are connected c. how heavy the atoms are d. what size the atoms are _____ 4. What do the backbones of some compounds have hundreds or thousands of? a. carbon atoms c. structural formulas b. carbon molecules d. acid ions HYDROCARBONS AND OTHER ORGANIC COM ...
Chemistry for Bio 11
... • Each orbit holds a determined number of electrons (first holds two, 2nd and 3rd hold eight ...
... • Each orbit holds a determined number of electrons (first holds two, 2nd and 3rd hold eight ...
International Arab Baccalaureate
... Organic Chemistry Amides are organic compounds which can be found in plants (coffee, tea, etc.) and in some medicinal drugs (paracetamol, penicillin, etc.). ...
... Organic Chemistry Amides are organic compounds which can be found in plants (coffee, tea, etc.) and in some medicinal drugs (paracetamol, penicillin, etc.). ...
Number of carbons
... • Poly means “many” • So it’s a large molecule made up of a series of “ethenes” (C2H4) • Plastic in soda bottles, etc. made of long noodle-like chains of these units. ...
... • Poly means “many” • So it’s a large molecule made up of a series of “ethenes” (C2H4) • Plastic in soda bottles, etc. made of long noodle-like chains of these units. ...
Chapter 4: The Periodic Table
... Organic Compounds 4 To name hydrocarbons find the longest line of Cs for root name where ending = -ane, -ene or –yne for alkanes, alkenes or alkynes. The prefix matches the # of Cs in the line with numbers inserted to tell where branches are. ...
... Organic Compounds 4 To name hydrocarbons find the longest line of Cs for root name where ending = -ane, -ene or –yne for alkanes, alkenes or alkynes. The prefix matches the # of Cs in the line with numbers inserted to tell where branches are. ...
Organic Chemistry Basics
... Carbon to carbon bonds are strong but not unbreakable, making them good for structural components Carbon can also form double and triple bonds with itself Carbon can form straight or branched chains as well as rings ...
... Carbon to carbon bonds are strong but not unbreakable, making them good for structural components Carbon can also form double and triple bonds with itself Carbon can form straight or branched chains as well as rings ...
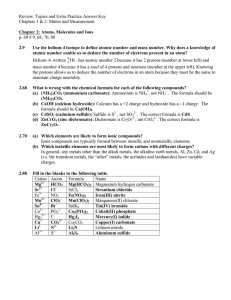
Name: Chapter 3 Reading Guide: Molecules, Compounds, and
... ____________________ molecular compounds, each with a _____________________ formula. Molecular compounds are composed of two or more _____________________. Generally, write the name of the element with the ______________________ group number first. If the two elements lie in the same group, then wri ...
... ____________________ molecular compounds, each with a _____________________ formula. Molecular compounds are composed of two or more _____________________. Generally, write the name of the element with the ______________________ group number first. If the two elements lie in the same group, then wri ...
Chemistry Lesson 40 Organic Chemistry
... g. Some compounds have more than one functional group – such as amino acids, which contain an amine (C-N) group and an organic acid (COOH) group. h. Naming organic compounds with functional groups involves using the alkane name, and adding the prefix/suffix for the functional group. ...
... g. Some compounds have more than one functional group – such as amino acids, which contain an amine (C-N) group and an organic acid (COOH) group. h. Naming organic compounds with functional groups involves using the alkane name, and adding the prefix/suffix for the functional group. ...
Presentation
... In the next few slides we will be looking at how to write formulas and names for three different types of chemical compounds: • 1. IONIC COMPOUNDS • 2. MOLECULAR COMPOUNDS • 3. ACIDS *Each type will have a set of rules that we must follow to correctly represent the substance. ...
... In the next few slides we will be looking at how to write formulas and names for three different types of chemical compounds: • 1. IONIC COMPOUNDS • 2. MOLECULAR COMPOUNDS • 3. ACIDS *Each type will have a set of rules that we must follow to correctly represent the substance. ...
Chemistry Quiz #1
... Neon is a noble gas and has a full outer shell. It is therefore satisfied and does not need to lose, gain or share any electrons. It will therefore form neither an ionic nor covalent bond. ...
... Neon is a noble gas and has a full outer shell. It is therefore satisfied and does not need to lose, gain or share any electrons. It will therefore form neither an ionic nor covalent bond. ...
transition metals
... DRAW the resonance structures in order to explain! Remember that a linkage isomer can bond to the metal ion from two distinctly different places on the molecule. And in order to have a bond – you need electrons!!! a. NO2-1 b. SO2 c. NO3-1 ...
... DRAW the resonance structures in order to explain! Remember that a linkage isomer can bond to the metal ion from two distinctly different places on the molecule. And in order to have a bond – you need electrons!!! a. NO2-1 b. SO2 c. NO3-1 ...
Chap2Jeopardy
... The nucleus of a helium atom contains: (a.) protons only. (b.) protons and neutrons. (c.) protons and electrons. (d.) electrons only. ...
... The nucleus of a helium atom contains: (a.) protons only. (b.) protons and neutrons. (c.) protons and electrons. (d.) electrons only. ...
Week 9
... elements or groups besides C and H. Oxygen, nitrogen, the halogens ( fluorine, chlorine, bromine, iodine ) phosphorus or sulfur. NO2 (nitrate) group OH (hydroxide) group PO4 (phosphate) group ...
... elements or groups besides C and H. Oxygen, nitrogen, the halogens ( fluorine, chlorine, bromine, iodine ) phosphorus or sulfur. NO2 (nitrate) group OH (hydroxide) group PO4 (phosphate) group ...
HW 2-1 Review Chap 2 Key
... Use the helium-4 isotope to define atomic number and mass number. Why does a knowledge of atomic number enable us to deduce the number of electrons present in an atom? Helium–4, written 42 He , has atomic number 2 because it has 2 protons (number at lower left) and mass number 4 because it has a tot ...
... Use the helium-4 isotope to define atomic number and mass number. Why does a knowledge of atomic number enable us to deduce the number of electrons present in an atom? Helium–4, written 42 He , has atomic number 2 because it has 2 protons (number at lower left) and mass number 4 because it has a tot ...
Homoaromaticity

Homoaromaticity in organic chemistry refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.The concept of homoaromaticity was pioneered by Saul Winstein in 1959, prompted by his studies of the “tris-homocyclopropenyl” cation. Since the publication of Winstein's paper, much research has been devoted to understanding and classifying these molecules, which represent an additional “class” of aromatic molecules included under the continuously broadening definition of aromaticity. To date, homoaromatic compounds are known to exist as cationic and anionic species, and some studies support the existence of neutral homoaromatic molecules, though these are less common. The 'homotropylium' cation (C8H9+) is perhaps the best studied example of a homoaromatic compound.