A review of Atoms
... Note that the electron is found outside of the nucleus.Here it is shown as a small dot circling the nucleus. There are the same number of electrons and protons in a neutral atom. ...
... Note that the electron is found outside of the nucleus.Here it is shown as a small dot circling the nucleus. There are the same number of electrons and protons in a neutral atom. ...
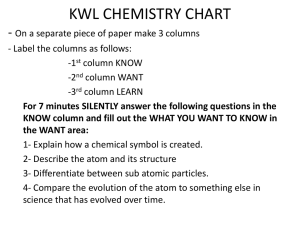
KWL chart and chem notes
... KNOW column and fill out the WHAT YOU WANT TO KNOW in the WANT area: 1- Explain how a chemical symbol is created. 2- Describe the atom and its structure 3- Differentiate between sub atomic particles. 4- Compare the evolution of the atom to something else in science that has evolved over time. ...
... KNOW column and fill out the WHAT YOU WANT TO KNOW in the WANT area: 1- Explain how a chemical symbol is created. 2- Describe the atom and its structure 3- Differentiate between sub atomic particles. 4- Compare the evolution of the atom to something else in science that has evolved over time. ...
CHAPTER 1 Practice Exercises 1.1 12.3 g Cd 1.3 26.9814 u 1.5
... atom, which does not affect the chemistry of the elements as much as the number of protons. ...
... atom, which does not affect the chemistry of the elements as much as the number of protons. ...
Atomic theory notes
... John Dalton: Early 1800s ~ discovered 4 parts of atomic theory 1. All elements are composed of atoms that cannot be divided 2. All atoms of the same element are exactly alike and have the same mass 3. An atom of one element cannot be changed into an atom of a different element. Atoms cannot be creat ...
... John Dalton: Early 1800s ~ discovered 4 parts of atomic theory 1. All elements are composed of atoms that cannot be divided 2. All atoms of the same element are exactly alike and have the same mass 3. An atom of one element cannot be changed into an atom of a different element. Atoms cannot be creat ...
Intensive Chemistry: the Structure of Matter
... • Robert Boyle later disproved this in the 1600’s http://atomictimeline.net/index.php for a full timeline of atom discoveries ...
... • Robert Boyle later disproved this in the 1600’s http://atomictimeline.net/index.php for a full timeline of atom discoveries ...
Name
... 20. How many protons and neutrons are contained in the nucleus of each of the following atoms? In an atom of each element, how many electrons are present? a. ...
... 20. How many protons and neutrons are contained in the nucleus of each of the following atoms? In an atom of each element, how many electrons are present? a. ...
6.1 Atoms and Elements
... in rows and columns based on patterns of similar properties. All matter is made up of elements. Gold, copper, and oxygen are examples of elements. Each element is made up of only one type of particle or atom. Gold and copper have different properties because they are made up of different types of at ...
... in rows and columns based on patterns of similar properties. All matter is made up of elements. Gold, copper, and oxygen are examples of elements. Each element is made up of only one type of particle or atom. Gold and copper have different properties because they are made up of different types of at ...
Atoms Introduction Notes and Vocabulary
... ELEMENT - matter that is made of only ONE type of atoms. Ex.: Iron, Silver, Carbon, Gold, Oxygen, and others located on the Periodic Table of Elements. PROTON – positively charged particle found in the nucleus of an atom / It has the same mass as a neutron NEUTRON- neutrally charged (no charge) part ...
... ELEMENT - matter that is made of only ONE type of atoms. Ex.: Iron, Silver, Carbon, Gold, Oxygen, and others located on the Periodic Table of Elements. PROTON – positively charged particle found in the nucleus of an atom / It has the same mass as a neutron NEUTRON- neutrally charged (no charge) part ...
Inside the Atom
... 2. Isotopes – atoms of the same element that have different numbers of neutrons 3. Mass number – number of protons plus number of neutrons 4. Atomic mass – the number found below the element symbol a. The average mass of an atom of an element b. The unit used for atomic mass is the atomic mass unit ...
... 2. Isotopes – atoms of the same element that have different numbers of neutrons 3. Mass number – number of protons plus number of neutrons 4. Atomic mass – the number found below the element symbol a. The average mass of an atom of an element b. The unit used for atomic mass is the atomic mass unit ...
Naming Ionic Compounds
... ** this is just like you learned for molecular compounds except you are not worried about the numbers of an element examples: NaCl – sodium chloride CaCl2 – calcium chloride Mg3N2 – magnesium nitride PbO – lead oxide ...
... ** this is just like you learned for molecular compounds except you are not worried about the numbers of an element examples: NaCl – sodium chloride CaCl2 – calcium chloride Mg3N2 – magnesium nitride PbO – lead oxide ...
Matter Review
... • In your notes, use your periodic tables to determine the following for the elements at the bottom. – The number of protons – The number of neutrons – The number of shells – The number of electrons on the outer most shell (valence electrons) – Draw the atoms for each ...
... • In your notes, use your periodic tables to determine the following for the elements at the bottom. – The number of protons – The number of neutrons – The number of shells – The number of electrons on the outer most shell (valence electrons) – Draw the atoms for each ...
CHAPTER 1: INTRODUCTION TO CHEMISTRY
... All groups except for the ones in the transition metals and metalloids all have similar characteristics ...
... All groups except for the ones in the transition metals and metalloids all have similar characteristics ...
(null): 096.AtomReview
... a. Energize atoms using heat or electricity b. Electrons jump up to higher energy “floors” c. Electrons jump back down to specific “floors” and emit light with specific energy = specific color d. Color of light allows us to calculate energies of electrons WITHOUT BEING ABLE TO “SEE” THEM 3. Show spe ...
... a. Energize atoms using heat or electricity b. Electrons jump up to higher energy “floors” c. Electrons jump back down to specific “floors” and emit light with specific energy = specific color d. Color of light allows us to calculate energies of electrons WITHOUT BEING ABLE TO “SEE” THEM 3. Show spe ...
File - Mrs. Riggs Online
... Electrons arranged in concentric layers that surround the nucleus called electron shells/energy levels/clouds/orbitals: ...
... Electrons arranged in concentric layers that surround the nucleus called electron shells/energy levels/clouds/orbitals: ...
Looking for Patterns in Chemical Reactivity
... A chemical bond forms between two atoms when their valence electrons form a stable arrangement together. ...
... A chemical bond forms between two atoms when their valence electrons form a stable arrangement together. ...
File
... the repeating pattern of their properties. • As new elements discovered, periodic table changed. • Number of protons in nucleus (atomic number), determines the chemical properties of an element. • Modern periodic tables are arranged in order of increasing atomic number. ...
... the repeating pattern of their properties. • As new elements discovered, periodic table changed. • Number of protons in nucleus (atomic number), determines the chemical properties of an element. • Modern periodic tables are arranged in order of increasing atomic number. ...
Chapter 1 Review Sheet
... 23. How was Mendeleev’s original periodic table of elements different from our current periodic table? Mendeleev’s table only had about 30 elements and it was ordered by increasing atomic mass, our current table has 118 elements and is ordered by increasing atomic number 24. How was Mendeleev’s orig ...
... 23. How was Mendeleev’s original periodic table of elements different from our current periodic table? Mendeleev’s table only had about 30 elements and it was ordered by increasing atomic mass, our current table has 118 elements and is ordered by increasing atomic number 24. How was Mendeleev’s orig ...
Chemistry Study Guide What is matter made of? Matter is anything
... Elements are unique, pure substances. Elements and the Periodic Table Elements are arranged in order of their atomic number. The atomic number of an element is the number of protons in the nucleus of an atom of that element. Every element has its own atomic number. The periodic table has horizontal ...
... Elements are unique, pure substances. Elements and the Periodic Table Elements are arranged in order of their atomic number. The atomic number of an element is the number of protons in the nucleus of an atom of that element. Every element has its own atomic number. The periodic table has horizontal ...
Atomic Structure
... • Democritus (460 – 370 BCE) • Among first to suggest existence of atoms • Believed atoms were indivisible and indestructible ...
... • Democritus (460 – 370 BCE) • Among first to suggest existence of atoms • Believed atoms were indivisible and indestructible ...
Atomic Structure Test Review Answer Key - Unit 1
... have the same 4 quantum numbers.) q. Heisenberg uncertainty principle- you cannot know the location and velocity of an electron at same time. r. atomic emission spectrumfrequencies of light emitted by an element. s. Frequency- number of wave cycles per second (s-1) t. Wavelength- distance from equiv ...
... have the same 4 quantum numbers.) q. Heisenberg uncertainty principle- you cannot know the location and velocity of an electron at same time. r. atomic emission spectrumfrequencies of light emitted by an element. s. Frequency- number of wave cycles per second (s-1) t. Wavelength- distance from equiv ...
Models of the Atom Intro
... blocks of all matter. • The periodic table is a list of all of the elements that can build matter. It’s a little like the alphabet of chemistry. • The periodic table tells us several things… ...
... blocks of all matter. • The periodic table is a list of all of the elements that can build matter. It’s a little like the alphabet of chemistry. • The periodic table tells us several things… ...
Unit 4 – Atomic Structure Study Guide
... Dalton considered atoms to be whole and indivisible, that is, only whole atoms can be combined to form compounds. In the above formula, there are 1.5 Mg atoms, which is not possible based upon the indivisibility of an atom. 4. Complete the following table on the subatomic particles. PARTICLE Proto ...
... Dalton considered atoms to be whole and indivisible, that is, only whole atoms can be combined to form compounds. In the above formula, there are 1.5 Mg atoms, which is not possible based upon the indivisibility of an atom. 4. Complete the following table on the subatomic particles. PARTICLE Proto ...
Periodic Table
... g. most are solids, malleable and ductile h. high melting and boiling points 2. Non-metals a. right of the staircase b. 5-8 electrons in their outermost energy level c. electron acceptors: gain e- ...
... g. most are solids, malleable and ductile h. high melting and boiling points 2. Non-metals a. right of the staircase b. 5-8 electrons in their outermost energy level c. electron acceptors: gain e- ...