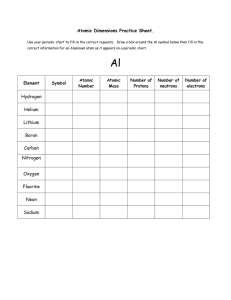
Review of Major Concepts Taught in Grade 9 Chemistry
... Valence electrons are the electrons found in the outermost electron orbit or shell. ...
... Valence electrons are the electrons found in the outermost electron orbit or shell. ...
Darlington High School EDI Lesson Plan Teacher: L. Grooms
... PS2.1 Compare the subatomic particles, protons, neutrons and electrons in regard to the mass, location, and charge and explain how these particles affect the properties of an atom. PS 2.3 Explain the trends of the periodic table based on the elements’ valence electrons and atomic number. PS 2.4 Use ...
... PS2.1 Compare the subatomic particles, protons, neutrons and electrons in regard to the mass, location, and charge and explain how these particles affect the properties of an atom. PS 2.3 Explain the trends of the periodic table based on the elements’ valence electrons and atomic number. PS 2.4 Use ...
What does an elements atomic # tell us about the element?
... Smallest part of a compound that still has all the properties of that compound Compound can have properties entirely unlike the elements of which it is made ...
... Smallest part of a compound that still has all the properties of that compound Compound can have properties entirely unlike the elements of which it is made ...
Worksheet 2: 1-19-17 - Iowa State University
... 2. Which of the following is not part of Daltons Atomic Theory of Matter? a. atoms cannot be changed to other elements by chemical reactions b. atoms of the same element are identical to one another c. elements are composed of atoms d. multiple atoms of the same element can combine to form other ele ...
... 2. Which of the following is not part of Daltons Atomic Theory of Matter? a. atoms cannot be changed to other elements by chemical reactions b. atoms of the same element are identical to one another c. elements are composed of atoms d. multiple atoms of the same element can combine to form other ele ...
Physical Science Chapter 6 Study Guide Atomic Theory of Matter
... o Alpha decay—a nucleus emits a clump of two protons and two neutrons o Beta decay—a nucleus changes a neutron into a proton and ejects an electron o Gamma decay—a nucleus emits high-energy electromagnetic rays ...
... o Alpha decay—a nucleus emits a clump of two protons and two neutrons o Beta decay—a nucleus changes a neutron into a proton and ejects an electron o Gamma decay—a nucleus emits high-energy electromagnetic rays ...
The Periodic Table HL Page 1 of 3 G. Galvin Name: Periodic Table
... -list the numbers of electrons in each main energy level in atoms of numbers 1-20 -build up the electronic structure of the first 36 elements -derive the electronic configurations of ions of s- and p-block elements only -describe the arrangement of electrons in individual orbitals of p-block atom ...
... -list the numbers of electrons in each main energy level in atoms of numbers 1-20 -build up the electronic structure of the first 36 elements -derive the electronic configurations of ions of s- and p-block elements only -describe the arrangement of electrons in individual orbitals of p-block atom ...
Democritus 440 BCE
... atoms. Atoms are small particles that cannot be created, divided, or destroyed. • Atoms of the same element are alike, atoms of different elements are different ...
... atoms. Atoms are small particles that cannot be created, divided, or destroyed. • Atoms of the same element are alike, atoms of different elements are different ...
GOB 3ed Chapter 2 part 1
... Configuration of an Atom Rule [2] Each orbital holds a maximum of 2 electrons. Rule [3] When orbitals are equal in energy: •1 electron is added to each orbital until all of the orbitals are half-filled. •Then, the orbitals can be completely filled. ...
... Configuration of an Atom Rule [2] Each orbital holds a maximum of 2 electrons. Rule [3] When orbitals are equal in energy: •1 electron is added to each orbital until all of the orbitals are half-filled. •Then, the orbitals can be completely filled. ...
Atoms and the Periodic Table
... ◦ Electrons move in set paths around the nucleus. ◦ Each electron has a certain energy, and the path defines the electrons energy level. ...
... ◦ Electrons move in set paths around the nucleus. ◦ Each electron has a certain energy, and the path defines the electrons energy level. ...
Midterm Review.ppt - Chemistry R: 4(AE)
... 1. Ar, Kr, Ne, Xe 2. Kr, Xe, Ar, Ne 3. Ne, Ar, Kr, Xe 4. Xe, Kr, Ar, Ne ...
... 1. Ar, Kr, Ne, Xe 2. Kr, Xe, Ar, Ne 3. Ne, Ar, Kr, Xe 4. Xe, Kr, Ar, Ne ...
PP - myndrs.com
... the nucleus of an atom in orbits or shells. • Each orbit is a certain distance from the nucleus and contains a definite number of electrons. • The orbits are filled in a routine way: – First orbit: 2 electrons – Second orbit: 8 electrons – Third orbit: 8 electrons ...
... the nucleus of an atom in orbits or shells. • Each orbit is a certain distance from the nucleus and contains a definite number of electrons. • The orbits are filled in a routine way: – First orbit: 2 electrons – Second orbit: 8 electrons – Third orbit: 8 electrons ...
Structure of an Atom structure_of_atom
... the nucleus of an atom in orbits or shells. • Each orbit is a certain distance from the nucleus and contains a definite number of electrons. • The orbits are filled in a routine way: – First orbit: 2 electrons – Second orbit: 8 electrons – Third orbit: 8 electrons ...
... the nucleus of an atom in orbits or shells. • Each orbit is a certain distance from the nucleus and contains a definite number of electrons. • The orbits are filled in a routine way: – First orbit: 2 electrons – Second orbit: 8 electrons – Third orbit: 8 electrons ...
PS 2.2
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
chpt 11 and 12 notes with answers
... element that has all the element’s properties. Composed of PROTONS and NEUTRONS and (found in the nucleus) ELECTRONS that revolve swiftly around the nucleus. ...
... element that has all the element’s properties. Composed of PROTONS and NEUTRONS and (found in the nucleus) ELECTRONS that revolve swiftly around the nucleus. ...
Atomic Structure/Electrons
... 10. He discovered the electron and developed the “plum pudding” model of the atom. 11. His five postulates make up atomic theory. 12. His gold foil experiment led to his discovery of the nucleus. 13. He developed the planetary model of the atom, which described the light spectrum. 14. What is the sh ...
... 10. He discovered the electron and developed the “plum pudding” model of the atom. 11. His five postulates make up atomic theory. 12. His gold foil experiment led to his discovery of the nucleus. 13. He developed the planetary model of the atom, which described the light spectrum. 14. What is the sh ...
Physical Science Chapter 3 Test
... their properties will emerge in a regular pattern. 12. Because atoms of elements in the same group of the periodic table have the same number of ____________________, they have similar properties. 13. Some elements are highly ____________________ because their outermost energy levels are only partia ...
... their properties will emerge in a regular pattern. 12. Because atoms of elements in the same group of the periodic table have the same number of ____________________, they have similar properties. 13. Some elements are highly ____________________ because their outermost energy levels are only partia ...
CHAPTER 18 NOTES
... • 2 ways to show difference between isotopes: 1. name of element followed by mass # 2. write the symbol with the mass # and atomic # ...
... • 2 ways to show difference between isotopes: 1. name of element followed by mass # 2. write the symbol with the mass # and atomic # ...
notes - van Maarseveen
... Information in the periodic table – if you look at the square for each element, you will find two important numbers Number at the top = atomic number Number at the bottom = atomic mass Why are the atomic masses not always whole numbers? Some elements have different forms (known as isotopes) that hav ...
... Information in the periodic table – if you look at the square for each element, you will find two important numbers Number at the top = atomic number Number at the bottom = atomic mass Why are the atomic masses not always whole numbers? Some elements have different forms (known as isotopes) that hav ...