File
... form more than one compound: the ratios of the mass of one element in the first compound to its mass in the second compound, (as it combines with the same mass of the other element), can always be expressed as ratios of small whole numbers( ex: 1:3 or 2:5). ...
... form more than one compound: the ratios of the mass of one element in the first compound to its mass in the second compound, (as it combines with the same mass of the other element), can always be expressed as ratios of small whole numbers( ex: 1:3 or 2:5). ...
Blue File
... Bohr developed a model from the findings of their research which is the widely recognised model that we use today….. With each atom being made up of a number of sub – atomic particles : Protons and Positive (+ ve) Elctrons are Negative (- ve) and Neutrons are Neutral Each element in the Periodic Tab ...
... Bohr developed a model from the findings of their research which is the widely recognised model that we use today….. With each atom being made up of a number of sub – atomic particles : Protons and Positive (+ ve) Elctrons are Negative (- ve) and Neutrons are Neutral Each element in the Periodic Tab ...
Matter: A) Homogeneous Matter • Uniform and in 1 phase • Even
... Always written with empirical formula (lowest whole number ratio) Name the metal and then name the nonmetal with an “ide” (cation then anion) All ionic compounds since they have a metal and a nonmetal, are salts Ex: NaCl (Sodium Chloride), MgBr2 (Magnesium Bromide) Use stock system when ther ...
... Always written with empirical formula (lowest whole number ratio) Name the metal and then name the nonmetal with an “ide” (cation then anion) All ionic compounds since they have a metal and a nonmetal, are salts Ex: NaCl (Sodium Chloride), MgBr2 (Magnesium Bromide) Use stock system when ther ...
File
... extremely reactive • fluorine is the most reactive • Noble gases (Group 18) – extremely low chemical reactivity • ASSIGN p.g. 55 - #1,4,5 and p.g. 67 #3 ...
... extremely reactive • fluorine is the most reactive • Noble gases (Group 18) – extremely low chemical reactivity • ASSIGN p.g. 55 - #1,4,5 and p.g. 67 #3 ...
john dalton!! - Hawk Chemistry
... • He was born September 6th, 1766 in Eaglesfield in Cumberland. • He died on July 27th, ...
... • He was born September 6th, 1766 in Eaglesfield in Cumberland. • He died on July 27th, ...
Unit 2 Practice Exam exam_2p_08_matter
... 42. Why do atomic radii increase dramatically with each additional row of the periodic table? a. atomic nuclei become increasingly attractive as more protons are added. b. another energy level is utilized by the electrons. c. the energy required to remove an electron is reduced by shielding of inter ...
... 42. Why do atomic radii increase dramatically with each additional row of the periodic table? a. atomic nuclei become increasingly attractive as more protons are added. b. another energy level is utilized by the electrons. c. the energy required to remove an electron is reduced by shielding of inter ...
Matter Unit KUD
... Understand: 1. The atomic model is used to describe what an atom is made of and how it acts with other atoms. 2. In the atomic model protons and neutrons are found in the nucleus; electrons are found in a cloud outside the nucleus. 3. Atoms of any element are alike. Atoms of different elements are d ...
... Understand: 1. The atomic model is used to describe what an atom is made of and how it acts with other atoms. 2. In the atomic model protons and neutrons are found in the nucleus; electrons are found in a cloud outside the nucleus. 3. Atoms of any element are alike. Atoms of different elements are d ...
Chapter 4.1 Notes
... - suggested universe was made of indivisible units called atomos – means unable to be cut or divided - did not have any evidence - forgotten until 1700’s ...
... - suggested universe was made of indivisible units called atomos – means unable to be cut or divided - did not have any evidence - forgotten until 1700’s ...
Final Exam Review
... Ch.8 Covalent Bonding: pg. 256 #39,43,77,110, pg.261 #6 39. The melting point of a compound is 1240 ͒C. Is this compound most likely an ionic compound or a molecular compound? 43. Identify the phrases that generally apply to molecular compounds: A. contain metals and nonmetals B. are often gases or ...
... Ch.8 Covalent Bonding: pg. 256 #39,43,77,110, pg.261 #6 39. The melting point of a compound is 1240 ͒C. Is this compound most likely an ionic compound or a molecular compound? 43. Identify the phrases that generally apply to molecular compounds: A. contain metals and nonmetals B. are often gases or ...
Test 1
... The mass of an atom in amu is approximated as the number of photons plus the number of neutrons present in the nucleus. Atoms can be split into a nucleus and the electrons, and the electrons move around the nucleus. Different isotopes of an element contain different numbers of neutrons. The three pr ...
... The mass of an atom in amu is approximated as the number of photons plus the number of neutrons present in the nucleus. Atoms can be split into a nucleus and the electrons, and the electrons move around the nucleus. Different isotopes of an element contain different numbers of neutrons. The three pr ...
Atomic Structure
... An atom is the smallest building block of matter. Atoms are made of neutrons, protons and electrons. The nucleus of an atom is extremely small in comparison to the atom. If an atom was the size of the Houston Astrodome, then its nucleus would be the size of a pea. Scientists use the Periodic Table i ...
... An atom is the smallest building block of matter. Atoms are made of neutrons, protons and electrons. The nucleus of an atom is extremely small in comparison to the atom. If an atom was the size of the Houston Astrodome, then its nucleus would be the size of a pea. Scientists use the Periodic Table i ...
Chapter 3 – Atomic Structure - Mercer Island School District
... • French chemist Joseph Proust, 1799 – Law of Constant Composition – compounds contain the same elements always in the same proportions. ...
... • French chemist Joseph Proust, 1799 – Law of Constant Composition – compounds contain the same elements always in the same proportions. ...
Chapter 18: Atoms and Elements
... Use indirect measurement to determine the radius of a circle. Build models of atoms. Research one of the historical atomic models. Understand how atoms of each element differ. Describe the forces that hold an atom together. Use the concept of electron shells to arrange electrons in atomic ...
... Use indirect measurement to determine the radius of a circle. Build models of atoms. Research one of the historical atomic models. Understand how atoms of each element differ. Describe the forces that hold an atom together. Use the concept of electron shells to arrange electrons in atomic ...
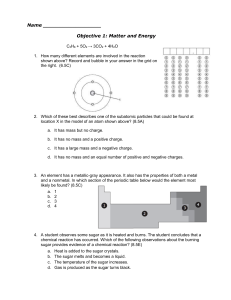
Name Objective 1: Matter and Energy C3H8 + 5O2 → 3CO2 + 4H2O
... 16. Which two compounds contain the same total number of atoms? (8.5D) a. C3H8 and C2H6 b. NO2 and KCl c. 2Li2S and Be4Cl2 d. 2CO and CO2 17. All of the following are indicators of a chemical change except — (8.5E) a. formation of a gas b. change in temperature c. change in the state of matter d. fo ...
... 16. Which two compounds contain the same total number of atoms? (8.5D) a. C3H8 and C2H6 b. NO2 and KCl c. 2Li2S and Be4Cl2 d. 2CO and CO2 17. All of the following are indicators of a chemical change except — (8.5E) a. formation of a gas b. change in temperature c. change in the state of matter d. fo ...
Physical Science Chapter 6 Study Guide Every element consists of
... o Every element consists of tiny particles called ____________ o All atoms of a particular element have the same ____________________ o Different elements have different properties because their atoms are different o Atoms of different elements can combine in specific ways to form ____________ o Che ...
... o Every element consists of tiny particles called ____________ o All atoms of a particular element have the same ____________________ o Different elements have different properties because their atoms are different o Atoms of different elements can combine in specific ways to form ____________ o Che ...
CP Chemistry First Semester Final Exam 1
... results from the attraction between (+) and (-) particles elements found left of the zig zag line on the periodic table Plum Pudding model of the atom any atom or molecule with a (-) charge occurs as atoms share valence electrons Gold Foil Experiment energy needed to move from one energy level to an ...
... results from the attraction between (+) and (-) particles elements found left of the zig zag line on the periodic table Plum Pudding model of the atom any atom or molecule with a (-) charge occurs as atoms share valence electrons Gold Foil Experiment energy needed to move from one energy level to an ...
History of Atomic Theory • Democritus: Atom meaning “unable to
... o Atoms of the same element are exactly alike. Atoms of different elements are different. o Atoms join together with other atoms to make new substances. JJ Thomson: “Plum pudding” Model (1897) o Discovered the electron. Rutherford: “Gold foil” experiment (1911) o Atom has a positive center (nucl ...
... o Atoms of the same element are exactly alike. Atoms of different elements are different. o Atoms join together with other atoms to make new substances. JJ Thomson: “Plum pudding” Model (1897) o Discovered the electron. Rutherford: “Gold foil” experiment (1911) o Atom has a positive center (nucl ...
c) C2 Glossary Topic 1
... The number of objects of a particular kind in a sample (shown as a percentage of the total number of objects) ...
... The number of objects of a particular kind in a sample (shown as a percentage of the total number of objects) ...
Atomic Structure and the Periodic Table
... Scientists once thought these metals were available only in tiny amounts on the Earth ...
... Scientists once thought these metals were available only in tiny amounts on the Earth ...
Matter
... an element’s atoms can vary. Isotopes are atoms of the same element that have different mass numbers and the same chemical properties. ...
... an element’s atoms can vary. Isotopes are atoms of the same element that have different mass numbers and the same chemical properties. ...