Chapter 1 OBJECTIVES
... Through technology the knowledge we have about cells growths by the minute. Before scientist were only able to see tiny spot and know a days they acknowledge it as the ubiquitous unit of a life. ...
... Through technology the knowledge we have about cells growths by the minute. Before scientist were only able to see tiny spot and know a days they acknowledge it as the ubiquitous unit of a life. ...
Chapter 8 Notes Photosynthesis
... • The high-energy electrons produced by chlorophyll are highly reactive and require a special “carrier.” • Think of a high-energy electron as being similar to a hot potato. If you wanted to move the potato from one place to another, you would use an oven mitt—a carrier—to transport it. • Plants use ...
... • The high-energy electrons produced by chlorophyll are highly reactive and require a special “carrier.” • Think of a high-energy electron as being similar to a hot potato. If you wanted to move the potato from one place to another, you would use an oven mitt—a carrier—to transport it. • Plants use ...
IB-Respiration-Notepacket
... molecules are produced per glucose a. Carbon dioxide = (How many total does that bring us to?_________) b. ATP= (How many total does that bring us to? _________) c. NADH = d. FADH = (How many total electron carrier molecules do we have all together so far? _________________________) e. Where does th ...
... molecules are produced per glucose a. Carbon dioxide = (How many total does that bring us to?_________) b. ATP= (How many total does that bring us to? _________) c. NADH = d. FADH = (How many total electron carrier molecules do we have all together so far? _________________________) e. Where does th ...
Student Study Guide
... The Krebs cycle completes the energy-yielding oxidation of organic molecules: a closer look (pp. 161166, FIGURES 9.11, 9.12) The conversion of pyruvate to acetyl CoA links glycolysis to the Krebs cycle. The twocarbon acetate of acetyl CoA joins the four-carbon oxaloacetate to form the six-carbon cit ...
... The Krebs cycle completes the energy-yielding oxidation of organic molecules: a closer look (pp. 161166, FIGURES 9.11, 9.12) The conversion of pyruvate to acetyl CoA links glycolysis to the Krebs cycle. The twocarbon acetate of acetyl CoA joins the four-carbon oxaloacetate to form the six-carbon cit ...
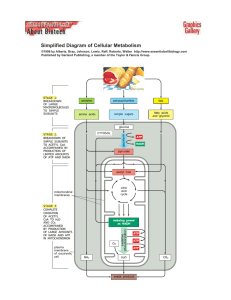
Simplified Diagram of Cellular Metabolism
... Published by Garland Publishing, a member of the Taylor & Francis Group. ...
... Published by Garland Publishing, a member of the Taylor & Francis Group. ...
fermentation
... When no air is present or readily available, a cell must still create ATP so it can perform other functions of life. This is where fermentation comes in to play. Glycolysis is the only reaction in aerobic respiration that requires no oxygen to move forward; it requires only a constant source of NAD+ ...
... When no air is present or readily available, a cell must still create ATP so it can perform other functions of life. This is where fermentation comes in to play. Glycolysis is the only reaction in aerobic respiration that requires no oxygen to move forward; it requires only a constant source of NAD+ ...
Cellular Respiration
... All NADH and FADH2 converted to ATP during this stage of cellular respiration. Each NADH converts to 3 ATP. Each FADH2 converts to 2 ATP (enters the ETC at a lower level than NADH). ...
... All NADH and FADH2 converted to ATP during this stage of cellular respiration. Each NADH converts to 3 ATP. Each FADH2 converts to 2 ATP (enters the ETC at a lower level than NADH). ...
103 final review worksheet
... 89. State whether each of the following activates or inhibits the citric acid cycle: ...
... 89. State whether each of the following activates or inhibits the citric acid cycle: ...
Chapter 9 - Slothnet
... with no release of CO2 • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt • Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce ...
... with no release of CO2 • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt • Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce ...
Gupta 2014 Credit: Google Images for the pictures Chapter 1
... standard nomenclature for noncomplexes.) 2. Within each complex (neutral or ion), name all ligands before the metal. -Name ligands in alphabetical order -If more than one of the same ligand is present, use a numerical prefix: di, tri, tetra, penta, hexa, … -Ignore numerical prefixes when alphabetizi ...
... standard nomenclature for noncomplexes.) 2. Within each complex (neutral or ion), name all ligands before the metal. -Name ligands in alphabetical order -If more than one of the same ligand is present, use a numerical prefix: di, tri, tetra, penta, hexa, … -Ignore numerical prefixes when alphabetizi ...
Energy systems.
... Fuel and energy for this comes from CHO, Fats, Proteins and Creatine phosphate. These fuel sources resynthesise the free Phosphate molecule (Pi) back to the ADP to reform ATP. ...
... Fuel and energy for this comes from CHO, Fats, Proteins and Creatine phosphate. These fuel sources resynthesise the free Phosphate molecule (Pi) back to the ADP to reform ATP. ...
Energy systems.
... Fuel and energy for this comes from CHO, Fats, Proteins and Creatine phosphate. These fuel sources resynthesise the free Phosphate molecule (Pi) back to the ADP to reform ATP. ...
... Fuel and energy for this comes from CHO, Fats, Proteins and Creatine phosphate. These fuel sources resynthesise the free Phosphate molecule (Pi) back to the ADP to reform ATP. ...
Lecture 3 Nutrient Roles in Bioenergetics
... Role of Oxygen in Energy Metabolism • 3 conditions for ATP re-synthesis using energy from macronutrients – Cond. 1: Availability of reduced NADH & FADH2 in tissues – Cond. 2: Presence of oxidizing agent O2 in the tissues – Cond. 3: Sufficient concentration of the enzymes & mitochondria in the ti ...
... Role of Oxygen in Energy Metabolism • 3 conditions for ATP re-synthesis using energy from macronutrients – Cond. 1: Availability of reduced NADH & FADH2 in tissues – Cond. 2: Presence of oxidizing agent O2 in the tissues – Cond. 3: Sufficient concentration of the enzymes & mitochondria in the ti ...
Ch. 2-4 Review
... a. The primary structure is the sequence of amino acids. b. Alpha helices and beta sheets are examples of secondary structure. c. Side chains (R-groups) of amino acids can be hydrophilic or hydrophobic. d. Proteins made of two or more polypeptide chains have quaternary structure. e. All statements a ...
... a. The primary structure is the sequence of amino acids. b. Alpha helices and beta sheets are examples of secondary structure. c. Side chains (R-groups) of amino acids can be hydrophilic or hydrophobic. d. Proteins made of two or more polypeptide chains have quaternary structure. e. All statements a ...
(key)
... During Photosynthesis what event occurs in the OEC? What does it stand for? C)j, 'J y'-"" 1»81~ J ~ ...
... During Photosynthesis what event occurs in the OEC? What does it stand for? C)j, 'J y'-"" 1»81~ J ~ ...
Krebs cycle - biology.org.uk
... 2 Citrate is decarboxylated (one molecule of CO 2 removed) and dehydrogenated (two hydrogen atoms removed) to form a five-carbon compound; and the hydrogen atoms are accepted by an NAD molecule, which gets reduced 3 The five-carbon compound is decarboxylated and dehydrogenated to give a four-carbon ...
... 2 Citrate is decarboxylated (one molecule of CO 2 removed) and dehydrogenated (two hydrogen atoms removed) to form a five-carbon compound; and the hydrogen atoms are accepted by an NAD molecule, which gets reduced 3 The five-carbon compound is decarboxylated and dehydrogenated to give a four-carbon ...
Chapter 8
... • Cells require a constant source of energy for life processes but keep only a small amount of ATP on hand. Cells can regenerate ATP as needed by using the energy stored in foods like glucose. • The energy stored in glucose by photosynthesis is released by cellular respiration and repackaged into t ...
... • Cells require a constant source of energy for life processes but keep only a small amount of ATP on hand. Cells can regenerate ATP as needed by using the energy stored in foods like glucose. • The energy stored in glucose by photosynthesis is released by cellular respiration and repackaged into t ...
Recovery
... 1. the return of the body to its pre-exercise state. 2. the extra volume of oxygen consumed during ...
... 1. the return of the body to its pre-exercise state. 2. the extra volume of oxygen consumed during ...
The citric acid cycle • Also known as the Kreb`s cycle
... • Energy of succinyl CoA is transferred (conserved) to GTP • SUBSTRATE LEVEL PHOSPHORYLATION: group transfer reaction • ONLY step where ATP is directly formed • All other ATP is produced by oxidative phosphorylation Oxid. Phosphor. is the oxidation of reduced cofactors (NADH, FADH2), to form ATP fro ...
... • Energy of succinyl CoA is transferred (conserved) to GTP • SUBSTRATE LEVEL PHOSPHORYLATION: group transfer reaction • ONLY step where ATP is directly formed • All other ATP is produced by oxidative phosphorylation Oxid. Phosphor. is the oxidation of reduced cofactors (NADH, FADH2), to form ATP fro ...
Cellular Respiration
... process that uses energy to extract energy (ATP) from macromolecules (glucose). Catabolic: Rxn that breaks molecules down Makes CO2 and H2O as well as energy (ATP) ...
... process that uses energy to extract energy (ATP) from macromolecules (glucose). Catabolic: Rxn that breaks molecules down Makes CO2 and H2O as well as energy (ATP) ...
9/29/2015 Chapter 9: CELLULAR RESPIRATION & FERMENTATION
... that is essentially “phase 2” of the catabolism of glucose: • pyruvate from glycolysis is first catabolized to acetyl-Coenzyme A before entering the CAC • all carbons from the original glucose will be completely oxidized to waste CO2 • more energy-rich e– in hydrogens will be captured by electron ca ...
... that is essentially “phase 2” of the catabolism of glucose: • pyruvate from glycolysis is first catabolized to acetyl-Coenzyme A before entering the CAC • all carbons from the original glucose will be completely oxidized to waste CO2 • more energy-rich e– in hydrogens will be captured by electron ca ...
Aerobic respiration - Wesleyan
... The reactions of glycolysis convert one molecule of glucose to two molecules of pyruvate for a net yield of two ATP An energy investment of ATP is required to start glycolysis Two ATP are used to split glucose and form 2 PGAL, each with one phosphate ...
... The reactions of glycolysis convert one molecule of glucose to two molecules of pyruvate for a net yield of two ATP An energy investment of ATP is required to start glycolysis Two ATP are used to split glucose and form 2 PGAL, each with one phosphate ...
Ch 9 Notes - Dublin City Schools
... • Electrons are transferred from NADH or FADH2 to the electron transport chain • Electrons are passed through a number of proteins including cytochromes (each with an iron atom) to O2 • The chain’s function is to break the large free-energy drop from food to O2 into smaller steps that release energ ...
... • Electrons are transferred from NADH or FADH2 to the electron transport chain • Electrons are passed through a number of proteins including cytochromes (each with an iron atom) to O2 • The chain’s function is to break the large free-energy drop from food to O2 into smaller steps that release energ ...
Application of Hard-Soft Acid-Base
... Hard-Soft Acid-Base (HSAB) Theory – Ralph G. Pearson (1963) – “Hard acids prefer to associate with hard bases, and soft acids prefer to associate with soft bases.” ...
... Hard-Soft Acid-Base (HSAB) Theory – Ralph G. Pearson (1963) – “Hard acids prefer to associate with hard bases, and soft acids prefer to associate with soft bases.” ...
DARK REACTIONS energy utilization The Calvin Cycle
... NIGHT Perform PEP carboxylase reaction at night (CO2 assimilation) accumulate malate to high concentration in central vacuole use sugar oxidation/catabolism to power (NADH and ATP) carbon fixation DAY Perform “light” reactions during the day mostly cyclic e- flow to produce ATP (low O2) decarboxylat ...
... NIGHT Perform PEP carboxylase reaction at night (CO2 assimilation) accumulate malate to high concentration in central vacuole use sugar oxidation/catabolism to power (NADH and ATP) carbon fixation DAY Perform “light” reactions during the day mostly cyclic e- flow to produce ATP (low O2) decarboxylat ...