answer key
... Epinephrine binding to its receptor stimulates production of cAMP and therefore promotes phosphorylation catalyzed by cAPK. When cAMP phosphodiesterase is inhibited, [cAMP] remains high and thereby prolongs the effects of epinephrine. Glycolysis is stimulated and produces more energy beyond what wou ...
... Epinephrine binding to its receptor stimulates production of cAMP and therefore promotes phosphorylation catalyzed by cAPK. When cAMP phosphodiesterase is inhibited, [cAMP] remains high and thereby prolongs the effects of epinephrine. Glycolysis is stimulated and produces more energy beyond what wou ...
I. ATP is Universal
... e) Phosphate groups are added to the C3 molecules. f) This stimulates the synthesis of ATP via substrate level ATP synthesis. An enzyme passes a high-energy phosphate to ADP. This is an example of the coupling of an energy-releasing reaction to an energy-requiring one. g) Oxidation of the resultant ...
... e) Phosphate groups are added to the C3 molecules. f) This stimulates the synthesis of ATP via substrate level ATP synthesis. An enzyme passes a high-energy phosphate to ADP. This is an example of the coupling of an energy-releasing reaction to an energy-requiring one. g) Oxidation of the resultant ...
Electron Transport Chain
... What is Cellular respiration and Anaerobic Fermentation and what are the differences between them. What are the four steps of aerobic cellular respiration, what happens in each step, what are the starting molecules, what comes out of each step, where in the cell does each step occur, how many AT ...
... What is Cellular respiration and Anaerobic Fermentation and what are the differences between them. What are the four steps of aerobic cellular respiration, what happens in each step, what are the starting molecules, what comes out of each step, where in the cell does each step occur, how many AT ...
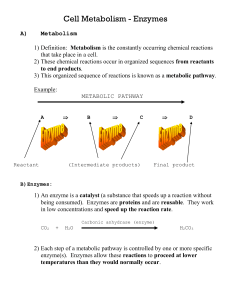
Enzymes - Kevan Kruger
... Review of LOCK AND KEY According to this analogy, an enzyme acts like a key by combining with a specific substrate and “unlocking” the substrate for further activity of the cell. This is a useful analogy because the key (enzyme) must have the correct shape to fit the lock (substrate). After the loc ...
... Review of LOCK AND KEY According to this analogy, an enzyme acts like a key by combining with a specific substrate and “unlocking” the substrate for further activity of the cell. This is a useful analogy because the key (enzyme) must have the correct shape to fit the lock (substrate). After the loc ...
Enzymes
... speed of a chemical reaction by lowering the energy requirement • Although a catalyst influences a chemical reaction, it is not itself permanently changed, nor does it cause the reaction to occur. • A catalyst can increase the rate of a chemical reaction but cannot cause the reaction . ...
... speed of a chemical reaction by lowering the energy requirement • Although a catalyst influences a chemical reaction, it is not itself permanently changed, nor does it cause the reaction to occur. • A catalyst can increase the rate of a chemical reaction but cannot cause the reaction . ...
Mock Exam 2 BY 123 – Dr. Biga Supplemental Instruction 1. Which
... A) Substrate level phosphorylation involves the transfer of a phosphate group directly from an organic molecule to ADP using an enzyme and oxidative phosphorylation uses chemiosmosis and ATP synthase B) Oxidative phosphorylation takes place in cellular respiration and substrate level phosphorylation ...
... A) Substrate level phosphorylation involves the transfer of a phosphate group directly from an organic molecule to ADP using an enzyme and oxidative phosphorylation uses chemiosmosis and ATP synthase B) Oxidative phosphorylation takes place in cellular respiration and substrate level phosphorylation ...
Chapter 8 THE ENERGY CONSUMING PROCESS OF RESPIRATION
... organisms can also produce ATP through chemical pathways that degrade (take apart) food molecules. The main degradative pathway requires free oxygen and is called (6) ______________. There are three stages of aerobic respiration. In the first stage, (7) ______________, glucose is partially degraded ...
... organisms can also produce ATP through chemical pathways that degrade (take apart) food molecules. The main degradative pathway requires free oxygen and is called (6) ______________. There are three stages of aerobic respiration. In the first stage, (7) ______________, glucose is partially degraded ...
File - Wk 1-2
... 3. Describe the pathways involved in energy metabolism: glycolysis, gluconeogenesis, beta-oxidation, amino acid breakdown, TCA cycle and electron transport chain. For each, include the cellular location, the major organs in which each pathway is active and the effect of starvation or flux of substra ...
... 3. Describe the pathways involved in energy metabolism: glycolysis, gluconeogenesis, beta-oxidation, amino acid breakdown, TCA cycle and electron transport chain. For each, include the cellular location, the major organs in which each pathway is active and the effect of starvation or flux of substra ...
Presentation
... active site side chain) • There are many naturally-occurring and synthetic irreversible inhibitors • These inhibitors can be used to identify the amino acid residues at enzyme active sites • Incubation of I with enzyme results in loss of activity ...
... active site side chain) • There are many naturally-occurring and synthetic irreversible inhibitors • These inhibitors can be used to identify the amino acid residues at enzyme active sites • Incubation of I with enzyme results in loss of activity ...
Rubisco
... Animals do not have these following enzymes so they can not perform photosynthesis: Sedoheptulose 1,7-bisphosphatase ribulose 5-phosphate kinase rubisco ...
... Animals do not have these following enzymes so they can not perform photosynthesis: Sedoheptulose 1,7-bisphosphatase ribulose 5-phosphate kinase rubisco ...
Notes: Enzymes
... Lactose intolerance develops when the body has difficulty digesting whole and skim milk and other dairy products. Lactose is a milk sugar and like most sugars, it is broken down by enzymes in the intestinal tract so it can be absorbed as an energy source. The enzyme that breaks down lactose is calle ...
... Lactose intolerance develops when the body has difficulty digesting whole and skim milk and other dairy products. Lactose is a milk sugar and like most sugars, it is broken down by enzymes in the intestinal tract so it can be absorbed as an energy source. The enzyme that breaks down lactose is calle ...
Cell Respiration
... As they are passed along the chain, the energy carried by these electrons is transformed in the mitochondrion into a form that can be used to synthesize ATP via oxidative phosphorylation. ...
... As they are passed along the chain, the energy carried by these electrons is transformed in the mitochondrion into a form that can be used to synthesize ATP via oxidative phosphorylation. ...
chapter 9 cellular respiration: harvesting chemical energy
... As they are passed along the chain, the energy carried by these electrons is transformed in the mitochondrion into a form that can be used to synthesize ATP via oxidative phosphorylation. ...
... As they are passed along the chain, the energy carried by these electrons is transformed in the mitochondrion into a form that can be used to synthesize ATP via oxidative phosphorylation. ...
The Central Role of Acetyl-CoA
... or a gland in one part of the body that transmit messages that affect cells in other parts of the organism. • Important hormones in human metabolism include: o Ghrelin - the hunger-stimulating hormone o Leptin - the satiety (full-feeling) hormone o Glucagon - the stored glucose releasing hormone o I ...
... or a gland in one part of the body that transmit messages that affect cells in other parts of the organism. • Important hormones in human metabolism include: o Ghrelin - the hunger-stimulating hormone o Leptin - the satiety (full-feeling) hormone o Glucagon - the stored glucose releasing hormone o I ...
Chapter 9 outline
... Oxidative phosphorylation – Is driven by the electron transport chain – Generates ATP ...
... Oxidative phosphorylation – Is driven by the electron transport chain – Generates ATP ...
Food Biotechnology Dr. Tarek Elbashiti 7. Metabolic Engineering of
... • From reasoning based on metabolic pathways structure, rerouting a carbon source to produce a desired amino acid should start by increasing the availability of precursor metabolites, energy, and reducing equivalents used in its synthesis. • Central metabolic pathways meet these criteria, and theref ...
... • From reasoning based on metabolic pathways structure, rerouting a carbon source to produce a desired amino acid should start by increasing the availability of precursor metabolites, energy, and reducing equivalents used in its synthesis. • Central metabolic pathways meet these criteria, and theref ...
Food Biotechnology Dr. Tarek Elbashiti
... • From reasoning based on metabolic pathways structure, rerouting a carbon source to produce a desired amino acid should start by increasing the availability of precursor metabolites, energy, and reducing equivalents used in its synthesis. • Central metabolic pathways meet these criteria, and theref ...
... • From reasoning based on metabolic pathways structure, rerouting a carbon source to produce a desired amino acid should start by increasing the availability of precursor metabolites, energy, and reducing equivalents used in its synthesis. • Central metabolic pathways meet these criteria, and theref ...
How Enzymes Are Named - Our biological products and solutions
... Enzymes are catalysts Enzymes are capable of performing these tasks because, unlike food proteins such as case in egg albumin, gelatine or soya protein, they are catalysts. This means that by their mere presence, and without being consumed in the process, enzymes can speed up chemical processes that ...
... Enzymes are catalysts Enzymes are capable of performing these tasks because, unlike food proteins such as case in egg albumin, gelatine or soya protein, they are catalysts. This means that by their mere presence, and without being consumed in the process, enzymes can speed up chemical processes that ...
K - UCLA Chemistry and Biochemistry
... (1 ATP, 1 GTP)—these first two reactions are that expensive! ...
... (1 ATP, 1 GTP)—these first two reactions are that expensive! ...
File
... that the rate of ATP production by anaerobic glycolysis can be up to 100 times faster than that of oxidative phosphorylation, but much glucose is consumed and the end product, ethanol, still has a lot of potential chemical energy. When mammalian muscle tissues are rapidly using ATP, they can regener ...
... that the rate of ATP production by anaerobic glycolysis can be up to 100 times faster than that of oxidative phosphorylation, but much glucose is consumed and the end product, ethanol, still has a lot of potential chemical energy. When mammalian muscle tissues are rapidly using ATP, they can regener ...
6-APA - Teknologi Industri Pertanian
... properties, does not interact with the active components, and its sweet cool taste masks the unpleasant taste of many drugs ...
... properties, does not interact with the active components, and its sweet cool taste masks the unpleasant taste of many drugs ...
Cellular respiration
... glucose to produce fructose bisphosphate – Fructose bisphosphate is broken down into two G3P molecules – During the energy harvesting stage, the two G3P molecules are converted into two pyruvate molecules, resulting in four ATP and two NADH molecules ...
... glucose to produce fructose bisphosphate – Fructose bisphosphate is broken down into two G3P molecules – During the energy harvesting stage, the two G3P molecules are converted into two pyruvate molecules, resulting in four ATP and two NADH molecules ...
Slides - Websupport1
... Cells and Mitochondria • Cells provide small organic molecules for their mitochondria • Mitochondria produce ATP that is used by the cell to perform cellular functions i.e. cells feed mitochondria nutrient and in return mitochondria provide the cells with energy (ATP). ...
... Cells and Mitochondria • Cells provide small organic molecules for their mitochondria • Mitochondria produce ATP that is used by the cell to perform cellular functions i.e. cells feed mitochondria nutrient and in return mitochondria provide the cells with energy (ATP). ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.