* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Enzymes - Kevan Kruger
Biochemical cascade wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Inositol-trisphosphate 3-kinase wikipedia , lookup
Restriction enzyme wikipedia , lookup
Alcohol dehydrogenase wikipedia , lookup
Beta-lactamase wikipedia , lookup
Transferase wikipedia , lookup
Lactoylglutathione lyase wikipedia , lookup
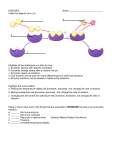
Cell Metabolism - Enzymes A) Metabolism 1) Definition: Metabolism is the constantly occurring chemical reactions that take place in a cell. 2) These chemical reactions occur in organized sequences from reactants to end products. 3) This organized sequence of reactions is known as a metabolic pathway. Example: METABOLIC PATHWAY A Reactant B C (Intermediate products) D Final product B) Enzymes: 1) An enzyme is a catalyst (a substance that speeds up a reaction without being consumed). Enzymes are proteins and are reusable. They work in low concentrations and speed up the reaction rate. Carbonic anhydrase (enzyme) CO2 + H2O H2CO3 2) Each step of a metabolic pathway is controlled by one or more specific enzyme(s). Enzymes allow these reactions to proceed at lower temperatures than they would normally occur. 3) The reactant(s) that an enzyme acts upon is known as the substrate(s). Enzymes work by forming a very temporary complex with the substrate. The shape of the substrate changes, and it becomes more reactive. Example (see p.103): Enzyme + Substrate Enzyme-Substrate Complex Enzyme + Product C) Enzyme Structures 1) Enzymes are large globular proteins with very specific 3-D shapes (tertiary structure). 2) Enzymes have grooves (or pockets), which may contain chemically functional groups. These areas are called active sites and this is where the substrate attaches. LOCK AND KEY THEORY OF ENZYME ACTION A. In order for a reaction to occur, the reactants (substrates) must be brought close together. B. The substrates bond to the active site on the enzyme, and are brought close together. Sometimes the active site changes shape to bring the substrates together. C. The reaction occurs and the product(s) are released. The enzyme goes back to its normal tertiary configuration. (shape) COMPLEX Review of LOCK AND KEY According to this analogy, an enzyme acts like a key by combining with a specific substrate and “unlocking” the substrate for further activity of the cell. This is a useful analogy because the key (enzyme) must have the correct shape to fit the lock (substrate). After the lock has been opened (reaction takes place) the key (enzyme) is freed and unchanged so that it may be used repeatedly in the same manner. The portion of the enzyme that is involved in the reaction is called the active site. 3) When the substrate attaches to the enzyme, this causes stress in the substrate, which will cause: a) a substrate to break apart (in a degradative reaction). Another word for this is CATABOLISM: breaking big molecules in to smaller ones. b) two substrates to form a bond (in a synthetic reaction). Another word for this is ANABOLISM: making big molecules from small. Note: ANABOLISM and CATABOLISM make up METABOLISM. 4) Specific groove shapes and chemical groups in an active site means that enzymes can only bond with one specific substrate or reactant. D) Enzyme Action 1) Enzymes operate by lowering the energy of activation needed for a reaction to occur. 2) Usually, heat is used to speed up chemical reactions by increasing the number of collisions that occur between reactants. Excessive heat, however, destroys the tertiary structure of protein (denatures) and therefore cannot be used to speed up reactions within living organisms. 3) Enzymes act as a CATALYST and are not consumed in a reaction. This means that they can be used over and over again. E) Factors Affecting Reaction Rate With Enzymes 1) Concentration: The amount of enzyme and/or substrate available to react can affect enzyme activity. a) The reaction speeds up if there are high concentrations of substrate, and it slows down if the [substrate] are depleted Rate of Reaction (product per unit of time) Time b) The reaction speeds up if there are high concentrations of enzyme, and it slows down if the [enzyme] are depleted. Rate of Reaction (product per unit of time) Time 2) Temperature: As temperature rises moderately, enzyme activity generally increases (according to the kinetic molecular theory). At a certain point, however, the enzyme becomes denatured (changes shape) by the increase in temperature and no longer functions properly. All enzymes have an optimal temperature (body temperature). Rate of Reaction (product per unit of time) 37C 3) pH: The 3D shape of an enzyme can be affected by pH and all enzymes have an optimal pH to work at (body pH, 6-8). Rate of Reaction (product per unit of time) pH 7 4) Inhibitors: Chemicals that interfere with the enzyme action. A metabolic product can feedback on a metabolic pathway to control how much product is made. Example: A enzyme # 1 B enzyme # 2 C enzyme #3 D (ie: Thyroxin) Product ‘D’ can temporarily attach to an active site on Enzyme #1. The enzyme will be denatured and will not bind to reactant A. This is an example of NEGATIVE FEEDBACK or FEEDBACK INHIBITION, and it will stop the reaction. When the concentration of the product F gets low again, the inhibitor (F) will fall away from enzyme #1, and the the metabolic pathway is reactivated. The enzyme #1 is free to react with substrate A again. There are two types of feedback inhibition: a) Competitive Inhibitors are chemicals that so closely resemble an enzyme’s normal substrate that it can attach to the enzymes active site. If the inhibitor occupies the active site of the enzyme, the reaction will be stopped. If the Inhibitor is removed, the enzyme will become active again. Example: SUBSTRATE or SUBSTRATE + Inhibitor Enzyme (E) Enzyme-Inhibitor Complex (EI) PRODUCT No Reaction! No Product! b) Non-competitive inhibitors are atoms or molecules that attach to an enzyme at an allosteric site (not the active site) and this denatures the enzyme. Non-competitive inhibitors will destroy an enzyme by permanently binding to the allosteric site. An example of this is heavy metals, such as lead, in the nervous system. Example: Feedback Inhibition and Control of Metabolic Rate Thyroxin, the hormone that controls the metabolic rate of all of the cells in your body, is produced by the thyroid gland in the neck. If the concentration Pituitary Gland of thyroxin in your body is high, your metabolic rate will be raised, and if thyroxin levels are low, your metabolic TSH rate will be low. The thyroid gland is stimulated to release thyroxin by a hormone produced Thyroid Gland in the pituitary gland called TSH (thyroid stimulating hormone). But the enzymes in cells of the pituitary that Thyroxin make TSH are inhibited by thyroxin. Therefore, if thyroxin levels are high, the pituitary stops producing TSH, Body Cells and if thyroxin levels are low, the (Metabolic Rate) pituitary makes the TSH. Thus, the metabolic rate of cells in your body are maintained by the feedback inhibition of an enzyme F) Co-enzymes Many enzymes are made up of 2 pieces. 1) The APOENZYME – the protein portion (inactive) 2) The CO-ENZYME – a non-protein portion When these join together, the enzyme becomes active. The Co-enzymes are often large molecules that the body cannot make on its own. Most co-enzymes are VITAMINS which we get from food. G) Cell Energy - ATP Most cell reactions (metabolism) require energy to occur. The energy ‘currency’ of cells is a molecule called ATP (Adenosine triphosphate) 1) Structure: made up of the nucleotide adenosine with 3 phosphates, the last two of which are held together by a high energy bond. 2) It takes energy to make this phosphate bond (~P), and energy is released when the bond is broken. A – P ~ P ~ P ATP (Adenosine Triphosphate) A – P ~ P + P + Energy ADP (Adenosine Diphosphate) 3) Where does the cell get ATP? It earns it from breaking food molecules apart and using the energy that is released to make ATP. This is what occurs when glucose is used during cellular respiration. C6H12O6 + 6O2 + ADP 6CO2 + 6H2O + ATP (energy) 4) When the cell needs energy for metabolism, it “cashes in” ATP, a metabolic reaction occurs, and the cell receives ADP as “change”.