New insight into pathogenesis of medical diseases
... immediate use or other forms that may be used in future. The foods possess stored energy. When we consume these foods, the digestive processes break them down into simple compounds that are absorbed into the body and transported to various cells. Energy in the body is available for immediate use in ...
... immediate use or other forms that may be used in future. The foods possess stored energy. When we consume these foods, the digestive processes break them down into simple compounds that are absorbed into the body and transported to various cells. Energy in the body is available for immediate use in ...
doc BIOC 311 Final Study Guide
... and E3 can't regenerate the disulfide bond because it's fully reduced and can't oxidize NAD+ in the presence of so much NADH. IV. CITRIC ACID CYCLE A. Functions: 1. Reduce NAD+ and FADH to generate ATP along the electron transport chain. 2. Generate organic intermediates for use in other biosyntheti ...
... and E3 can't regenerate the disulfide bond because it's fully reduced and can't oxidize NAD+ in the presence of so much NADH. IV. CITRIC ACID CYCLE A. Functions: 1. Reduce NAD+ and FADH to generate ATP along the electron transport chain. 2. Generate organic intermediates for use in other biosyntheti ...
Ch. 8 Review Sheet
... 25. In what cell structures do these reactions occur? A. I occurs in the stroma, II occurs in the cytoplasm B. I occurs in the cytoplasm, II occurs in the thylakoids C. I occurs in the thylakoids, II occurs in the stroma D. I occurs in the cytoplasm, II occurs in the stroma E. both occur in the cyto ...
... 25. In what cell structures do these reactions occur? A. I occurs in the stroma, II occurs in the cytoplasm B. I occurs in the cytoplasm, II occurs in the thylakoids C. I occurs in the thylakoids, II occurs in the stroma D. I occurs in the cytoplasm, II occurs in the stroma E. both occur in the cyto ...
How Enzymes Work - Manhasset Public Schools
... a) Organic- contains carbon and hydrogen a) Catalysts- affect the rate of chemical reactions ...
... a) Organic- contains carbon and hydrogen a) Catalysts- affect the rate of chemical reactions ...
Chapter 9: Cellular Respiration Notes
... • The transfer of electrons during chemical reactions releases energy stored in organic molecules • This released energy is ultimately used to synthesize ATP • Chemical reactions that transfer electrons between reactants are called oxidation-reduction reactions, or redox reactions • In oxidation, a ...
... • The transfer of electrons during chemical reactions releases energy stored in organic molecules • This released energy is ultimately used to synthesize ATP • Chemical reactions that transfer electrons between reactants are called oxidation-reduction reactions, or redox reactions • In oxidation, a ...
Amfep Fact Sheet on Enzymes from May 2015
... Enzymes are proteins - primary constituents of all living organisms. They act as catalysts. This means that they make biochemical reactions happen faster than they would otherwise. Without enzymes, those reactions simply would not occur or would run too slowly to sustain life. Many biochemical react ...
... Enzymes are proteins - primary constituents of all living organisms. They act as catalysts. This means that they make biochemical reactions happen faster than they would otherwise. Without enzymes, those reactions simply would not occur or would run too slowly to sustain life. Many biochemical react ...
Metabol Nutri-ClinEnz Med 2_6 Nov 2012
... serum, blood, urine etc.) are widely used. 3) direct measurement of enzyme protein concentration. This is limited due to very low concentrations of proteins. ...
... serum, blood, urine etc.) are widely used. 3) direct measurement of enzyme protein concentration. This is limited due to very low concentrations of proteins. ...
Lactic acid fermentation
... is usually done through an electron transport chain in a process called oxidative phosphorylation; however, this mechanism is not available without oxygen.[3][4] Instead, the NADH donates its extra electrons to the pyruvate molecules formed during glycolysis. Since the NADH has lost electrons, NAD+ ...
... is usually done through an electron transport chain in a process called oxidative phosphorylation; however, this mechanism is not available without oxygen.[3][4] Instead, the NADH donates its extra electrons to the pyruvate molecules formed during glycolysis. Since the NADH has lost electrons, NAD+ ...
Dehydrogenase Complexes of Corn (Zea mays L.) and Soybean
... grass-specific herbicides showed the following: (a) an accumulation of free sugars in mature leaves of the injured plant (1, 5), (b) the appearance of purple leaf coloration due to the accumulation of anthocyanin (1), (c) the simultaneous inhibition of respiration and lipid synthesis in cultured cel ...
... grass-specific herbicides showed the following: (a) an accumulation of free sugars in mature leaves of the injured plant (1, 5), (b) the appearance of purple leaf coloration due to the accumulation of anthocyanin (1), (c) the simultaneous inhibition of respiration and lipid synthesis in cultured cel ...
Oxidative Decarboxylation and Krebs Cycle
... Fates of Pyruvate Anaerobic Occurs in yeast and some bacteria ( intestinal flora ) ...
... Fates of Pyruvate Anaerobic Occurs in yeast and some bacteria ( intestinal flora ) ...
Chapter 5:Bioenergetics and oxidative phosphorylation Q1: why is
... Q3: Are NADH & FADH2 Produced in the mitochondria only? Q4: why does FADH2 produce 2ATP while NADH produce 3ATP? Q5: what are the site-specific inhibitors of the electron transport chain? Q6: Explain why NADH is oxidized by FMN? Q7: How is electron transport chain is coupled to oxidative phosphoryla ...
... Q3: Are NADH & FADH2 Produced in the mitochondria only? Q4: why does FADH2 produce 2ATP while NADH produce 3ATP? Q5: what are the site-specific inhibitors of the electron transport chain? Q6: Explain why NADH is oxidized by FMN? Q7: How is electron transport chain is coupled to oxidative phosphoryla ...
Bio 20 7.4 - Stirling School
... Yeast is added which will undergo fermentation. The bread will rise due to the release of Carbon dioxide. Alcohol is also produced Can you get drunk by eating bread? Alcohol is produced, but it evaporates upon baking. Don’t try to eat dough to get drunk!! It will make you ...
... Yeast is added which will undergo fermentation. The bread will rise due to the release of Carbon dioxide. Alcohol is also produced Can you get drunk by eating bread? Alcohol is produced, but it evaporates upon baking. Don’t try to eat dough to get drunk!! It will make you ...
Cellular Respiration
... 9.6: Glycolysis and the citric acid cycle connect to many other metabolic pathways • Catabolic pathways are versatile; they funnel electrons from many kinds of organic molecules (not just glucose!) into cellular respiration • Glycolysis accepts a wide range of carbohydrates • Proteins must first be ...
... 9.6: Glycolysis and the citric acid cycle connect to many other metabolic pathways • Catabolic pathways are versatile; they funnel electrons from many kinds of organic molecules (not just glucose!) into cellular respiration • Glycolysis accepts a wide range of carbohydrates • Proteins must first be ...
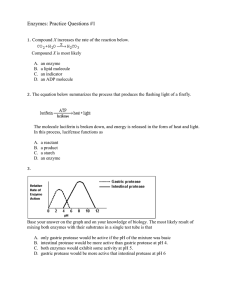
Enzymes: Practice Questions #1
... The graph shows the relative rates of action of four enzymes, A, B, C, and D. A solution with a pH of 6 contains enzyme C and its substrate. If a base is gradually added to this solution, the rate of action of enzyme C would most likely A. B. C. D. ...
... The graph shows the relative rates of action of four enzymes, A, B, C, and D. A solution with a pH of 6 contains enzyme C and its substrate. If a base is gradually added to this solution, the rate of action of enzyme C would most likely A. B. C. D. ...
Cell Size and Shape
... These two stages are preceded by an intermediate step in which pyruvic acid is converted to acetyl-CoA ...
... These two stages are preceded by an intermediate step in which pyruvic acid is converted to acetyl-CoA ...
Lecture 27
... In mammals, found in the liver and small intestine mucosa XO is a homodimer with FAD, two [2Fe-2S] clusters and a molybdopterin complex (Mo-pt) that cycles between Mol (VI) and Mol (IV) oxidation states. Final electron acceptor is O2 which is converted to H2O2 XO is cleaved into 3 segments. The uncl ...
... In mammals, found in the liver and small intestine mucosa XO is a homodimer with FAD, two [2Fe-2S] clusters and a molybdopterin complex (Mo-pt) that cycles between Mol (VI) and Mol (IV) oxidation states. Final electron acceptor is O2 which is converted to H2O2 XO is cleaved into 3 segments. The uncl ...
acid
... NADH, the removal of hydrogen (oxidation) causes dehydrogenation and we have also energy released in the form of Pi group, glyceric acid 1,3 diphosphate high energy compound. If glycolysis is aerobic, the NADH can be reoxidized (indirectly) by the mitochondrial electron transport chain, providing en ...
... NADH, the removal of hydrogen (oxidation) causes dehydrogenation and we have also energy released in the form of Pi group, glyceric acid 1,3 diphosphate high energy compound. If glycolysis is aerobic, the NADH can be reoxidized (indirectly) by the mitochondrial electron transport chain, providing en ...
Immobilization of Enzymes
... inactivated by heat (i.e., thermolabile), may be rendered heat-stable by attachment to inert polymeric supports. ...
... inactivated by heat (i.e., thermolabile), may be rendered heat-stable by attachment to inert polymeric supports. ...
Document
... large molecules to smaller ones that enter the bloodstream. • Stage 2: Degradation break down molecules to two- and three-carbon compounds. • Stage 3: Oxidation of small molecules in the citric acid cycle and electron transport provides ATP energy. ...
... large molecules to smaller ones that enter the bloodstream. • Stage 2: Degradation break down molecules to two- and three-carbon compounds. • Stage 3: Oxidation of small molecules in the citric acid cycle and electron transport provides ATP energy. ...
Glycolysis
... 5. Triose phosphate isomerase Salvages a Three-Carbon Fragment Glyceraldehyde 3-phosphate is on the direct pathway of glycolysis, whereas dihydroxyacetone phosphate is not. Unless a means exists to convert dihydroxyacetone phosphate into glyceraldehyde 3-phosphate, a three-carbon fragment useful fo ...
... 5. Triose phosphate isomerase Salvages a Three-Carbon Fragment Glyceraldehyde 3-phosphate is on the direct pathway of glycolysis, whereas dihydroxyacetone phosphate is not. Unless a means exists to convert dihydroxyacetone phosphate into glyceraldehyde 3-phosphate, a three-carbon fragment useful fo ...
Adding Enzymes To Dairy Diets
... here is the background required to understand the applicability of this technology: ...
... here is the background required to understand the applicability of this technology: ...
Cellular Respiration
... The electron transport chain is in the cristae of the mitochondrion Most of the chain’s components are proteins, which exist in multi-protein complexes The carriers alternate reduced and oxidized states as they accept and donate electrons Electrons drop in free energy as they go down the chain and a ...
... The electron transport chain is in the cristae of the mitochondrion Most of the chain’s components are proteins, which exist in multi-protein complexes The carriers alternate reduced and oxidized states as they accept and donate electrons Electrons drop in free energy as they go down the chain and a ...
factors_effecting_en..
... fastest. For most enzymes this is about pH 7-8 (physiological pH of most cells), but a few enzymes can work at extreme pH, such as protease enzymes in animal stomachs, which have an optimum of pH 1 The pH affects the charge of the amino acids at the active site, so the properties of the active site ...
... fastest. For most enzymes this is about pH 7-8 (physiological pH of most cells), but a few enzymes can work at extreme pH, such as protease enzymes in animal stomachs, which have an optimum of pH 1 The pH affects the charge of the amino acids at the active site, so the properties of the active site ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.