
Unit 2 - CST Personal Home Pages
... from organic compounds to electron carrier molecules and then to final electron acceptor molecules. The transfer of electrons releases energy that is used to convert ADP ATP. 3. by photophosporylation – occurs in photosynthetic cells only. Light energy is converted to ATP. ...
... from organic compounds to electron carrier molecules and then to final electron acceptor molecules. The transfer of electrons releases energy that is used to convert ADP ATP. 3. by photophosporylation – occurs in photosynthetic cells only. Light energy is converted to ATP. ...
NO OXYGEN!
... when oxygen is not available. For example, in muscle tissues during rapid and vigorous exercise, muscle cells may be depleted of oxygen. They then switch from respiration to fermentation. ...
... when oxygen is not available. For example, in muscle tissues during rapid and vigorous exercise, muscle cells may be depleted of oxygen. They then switch from respiration to fermentation. ...
LAB 6 – Fermentation & Cellular Respiration INTRODUCTION
... and inorganic phosphate (Pi) is exergonic and thus releases energy which cells can use to do any number of things. Once hydrolyzed, ATP can be regenerated from ADP and Pi, though this is endergonic and thus requires energy. The energy needed to regenerate ATP is obtained from “food”, whatever that m ...
... and inorganic phosphate (Pi) is exergonic and thus releases energy which cells can use to do any number of things. Once hydrolyzed, ATP can be regenerated from ADP and Pi, though this is endergonic and thus requires energy. The energy needed to regenerate ATP is obtained from “food”, whatever that m ...
biol 161 aerobic cellular respiration
... 1. How many ATP molecules formed from substrate-level phosphorylation during glycolysis? 2. How many ATP molecules formed from substrate-level phosphorylation during citric acid cycle? B. Oxidative phosphorylation means that ATP is produced from the combination of electron transport chain and chemio ...
... 1. How many ATP molecules formed from substrate-level phosphorylation during glycolysis? 2. How many ATP molecules formed from substrate-level phosphorylation during citric acid cycle? B. Oxidative phosphorylation means that ATP is produced from the combination of electron transport chain and chemio ...
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL
... with the hydrogen from methane to form water, the electrons of the covalent bonds are drawn closer to the oxygen. ° In effect, each oxygen atom has partially “gained” electrons, and so the oxygen molecule ...
... with the hydrogen from methane to form water, the electrons of the covalent bonds are drawn closer to the oxygen. ° In effect, each oxygen atom has partially “gained” electrons, and so the oxygen molecule ...
B324notesTheme 2
... PFK-1 is a tetrameric enzyme that exist in two conformational states termed R and T that are in equilibrium. ATP is both a substrate and an allosteric inhibitor of PFK-1. Each subunit has two ATP binding sites, a substrate site and an inhibitor site. The substrate site binds ATP equally well when th ...
... PFK-1 is a tetrameric enzyme that exist in two conformational states termed R and T that are in equilibrium. ATP is both a substrate and an allosteric inhibitor of PFK-1. Each subunit has two ATP binding sites, a substrate site and an inhibitor site. The substrate site binds ATP equally well when th ...
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL
... with the hydrogen from methane to form water, the electrons of the covalent bonds are drawn closer to the oxygen. In effect, each oxygen atom has partially “gained” electrons, and so the oxygen molecule ...
... with the hydrogen from methane to form water, the electrons of the covalent bonds are drawn closer to the oxygen. In effect, each oxygen atom has partially “gained” electrons, and so the oxygen molecule ...
Why Glycogen as an Energy Storage Molecule?
... - Buildup of substrate lactate (lactic acid) causing acidosis (rarely overt) - Hypoglycemia (low blood sugar) in undernourished people. Can lead to irreversible CNS damage ...
... - Buildup of substrate lactate (lactic acid) causing acidosis (rarely overt) - Hypoglycemia (low blood sugar) in undernourished people. Can lead to irreversible CNS damage ...
video slide
... glucose NADH electron transport chain proton-motive force ATP • About 40% of the energy in a glucose molecule is transferred to ATP during cellular respiration, making about 38 ATP ...
... glucose NADH electron transport chain proton-motive force ATP • About 40% of the energy in a glucose molecule is transferred to ATP during cellular respiration, making about 38 ATP ...
Overview of Metabolism Chapter
... Figure 5. Cellular respiration consists of three metabolic phases: glycolysis, the Krebs cycle, and the electron transport chain. Glycolysis occurs in the cytosol, but the Krebs cycle and electron transport chain occur inside the mitochondria. Electron carriers such as NADH produced during glycolysi ...
... Figure 5. Cellular respiration consists of three metabolic phases: glycolysis, the Krebs cycle, and the electron transport chain. Glycolysis occurs in the cytosol, but the Krebs cycle and electron transport chain occur inside the mitochondria. Electron carriers such as NADH produced during glycolysi ...
REGULATORY ENZYMES
... by Covalent Modifications • Another common regulatory mechanism is the reversible covalent modification of an enzyme. Phosphorylation, whereby a phosphate is transferred from an activated donor (usually ATP) to an amino acid on the regulatory enyme, is the most common example of this type of regulat ...
... by Covalent Modifications • Another common regulatory mechanism is the reversible covalent modification of an enzyme. Phosphorylation, whereby a phosphate is transferred from an activated donor (usually ATP) to an amino acid on the regulatory enyme, is the most common example of this type of regulat ...
File
... CN- attach to the –SH groups in the enzyme This destroys the disulfide bridges and thus changing the tertiary structure of the enzyme Change in the shape results in the change in the active site thus the substrate cannot bind and cytochrome c oxidase is nonfuctional. ...
... CN- attach to the –SH groups in the enzyme This destroys the disulfide bridges and thus changing the tertiary structure of the enzyme Change in the shape results in the change in the active site thus the substrate cannot bind and cytochrome c oxidase is nonfuctional. ...
Chapt 6
... The citric acid cycle completes the oxidation of organic molecules, generating many NADH and FADH2 molecules • The citric acid cycle • is also called the Krebs cycle (after the GermanBritish researcher Hans Krebs, who worked out much of this pathway in the 1930s), • completes the oxidation of organ ...
... The citric acid cycle completes the oxidation of organic molecules, generating many NADH and FADH2 molecules • The citric acid cycle • is also called the Krebs cycle (after the GermanBritish researcher Hans Krebs, who worked out much of this pathway in the 1930s), • completes the oxidation of organ ...
Enzyme structure and function
... 1. A catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. 2. An enzyme speeds up the rate of a specific reaction, without being used up. 3. What does each enzyme do? Complete the sentences about specific enzymes. a. Lipase br ...
... 1. A catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. 2. An enzyme speeds up the rate of a specific reaction, without being used up. 3. What does each enzyme do? Complete the sentences about specific enzymes. a. Lipase br ...
Lecture 33
... Glucose-6P dehydrogenase (G6PD)– enzyme catalyzing the first reaction in the pathway which converts glucose-6P to 6phosphogluconolactone. This reaction is the commitment step in the pathway and is feedback-inhibited by NADPH. Defects in glucose-6P dehydrogenase cause a dietary condition called favis ...
... Glucose-6P dehydrogenase (G6PD)– enzyme catalyzing the first reaction in the pathway which converts glucose-6P to 6phosphogluconolactone. This reaction is the commitment step in the pathway and is feedback-inhibited by NADPH. Defects in glucose-6P dehydrogenase cause a dietary condition called favis ...
Final Exam - UC Davis Plant Sciences
... b) How does the synthesis of ornithine proceed, taking into consideration that one reaction requires NAD+, another is catalyzed by a transaminase, and yet another produces ADP as one of its products (Note: all reactions have been discussed in class in a different context)? Draw the structures of the ...
... b) How does the synthesis of ornithine proceed, taking into consideration that one reaction requires NAD+, another is catalyzed by a transaminase, and yet another produces ADP as one of its products (Note: all reactions have been discussed in class in a different context)? Draw the structures of the ...
Properties of Enzymes
... arrangement of a.a. in the active site that participate in the bond making and bond breaking (These residues are called catalytic groups) The specificiting of an enzyme is determined by: (a) Functional groups of enzyme (specific a.a. side chains, metal ions, and coenzymes) (b) Functional groups of s ...
... arrangement of a.a. in the active site that participate in the bond making and bond breaking (These residues are called catalytic groups) The specificiting of an enzyme is determined by: (a) Functional groups of enzyme (specific a.a. side chains, metal ions, and coenzymes) (b) Functional groups of s ...
Cellular Respiration
... Cellular Respiration • A catabolic, exergonic, oxygen (O2) requiring process that uses energy extracted from macromolecules (glucose) to produce energy (ATP) and water (H2O). ...
... Cellular Respiration • A catabolic, exergonic, oxygen (O2) requiring process that uses energy extracted from macromolecules (glucose) to produce energy (ATP) and water (H2O). ...
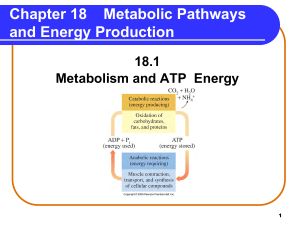
Metabolic Pathways and Energy Production
... • NADH (Complex I) oxidation for 3 ATPs. NADH + 3 ADP + 3Pi NAD+ + 3 ATP • FADH2 (Complex II) oxidation for 2 ATPs. FADH2 + 2 ADP + 2Pi FAD + 2 ATP ...
... • NADH (Complex I) oxidation for 3 ATPs. NADH + 3 ADP + 3Pi NAD+ + 3 ATP • FADH2 (Complex II) oxidation for 2 ATPs. FADH2 + 2 ADP + 2Pi FAD + 2 ATP ...
Chapter 6 Slides
... 6.9 The citric acid cycle completes the oxidation of organic molecules, generating many NADH and FADH2 molecules The citric acid cycle – is also called the Krebs cycle (after the German-British researcher Hans Krebs, who worked out much of this pathway in the 1930s), – completes the oxidation of ...
... 6.9 The citric acid cycle completes the oxidation of organic molecules, generating many NADH and FADH2 molecules The citric acid cycle – is also called the Krebs cycle (after the German-British researcher Hans Krebs, who worked out much of this pathway in the 1930s), – completes the oxidation of ...
JVB112 gluconeogenesis[1]
... reaction in the direction of pyruvate formation b. In muscle cells and erythrocytes, LDH usually runs this reaction in the direction of lactate formation c. The direction in which the reaction proceeds depends on I ) The ratios of NAD+ to NADH and lactate to pyruvate ii) The isozyme of LDH that is p ...
... reaction in the direction of pyruvate formation b. In muscle cells and erythrocytes, LDH usually runs this reaction in the direction of lactate formation c. The direction in which the reaction proceeds depends on I ) The ratios of NAD+ to NADH and lactate to pyruvate ii) The isozyme of LDH that is p ...
JVB112 gluconeogenesis[1]
... reaction in the direction of pyruvate formation b. In muscle cells and erythrocytes, LDH usually runs this reaction in the direction of lactate formation c. The direction in which the reaction proceeds depends on I ) The ratios of NAD+ to NADH and lactate to pyruvate ii) The isozyme of LDH that is p ...
... reaction in the direction of pyruvate formation b. In muscle cells and erythrocytes, LDH usually runs this reaction in the direction of lactate formation c. The direction in which the reaction proceeds depends on I ) The ratios of NAD+ to NADH and lactate to pyruvate ii) The isozyme of LDH that is p ...
(i) Enzymes are (1)
... A slows down all chemical reactions B speeds up a chemical reaction C prevents all chemical reactions taking place D has no effect on a chemical reaction (b) The diagrams show two sequences of six amino acids. Sequence 1 is found in an enzyme called catalase. ...
... A slows down all chemical reactions B speeds up a chemical reaction C prevents all chemical reactions taking place D has no effect on a chemical reaction (b) The diagrams show two sequences of six amino acids. Sequence 1 is found in an enzyme called catalase. ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.



















![JVB112 gluconeogenesis[1]](http://s1.studyres.com/store/data/000939420_1-ae0fa12f0b4eac306770097ba9ecae40-300x300.png)
![JVB112 gluconeogenesis[1]](http://s1.studyres.com/store/data/005255251_1-e457e3f80be2f5d8ecf577d50c416034-300x300.png)
