the Overview - The United Mitochondrial Disease
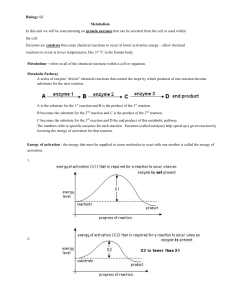
... As foodstuffs pass down the alimentary canal they are reacted on by sets of enzymes that break down carbohydrates to glucose, fats to fatty acids, and proteins to amino acids, which then circulate in the blood stream. Entry of these nutrients into cells in various tissues is under control of severa ...
... As foodstuffs pass down the alimentary canal they are reacted on by sets of enzymes that break down carbohydrates to glucose, fats to fatty acids, and proteins to amino acids, which then circulate in the blood stream. Entry of these nutrients into cells in various tissues is under control of severa ...
Chapter 8 Cellular Respiration Dr. Harold Kay Njemanze 8.1
... carbohydrates and other metabolites with the resultant buildup of ATP. 2. Cellular respiration consumes oxygen and produces CO2; because oxygen is required, cellular respiration is aerobic. 3. Cellular respiration usually involves the complete breakdown of glucose into CO2 and H2O. 4. The net equati ...
... carbohydrates and other metabolites with the resultant buildup of ATP. 2. Cellular respiration consumes oxygen and produces CO2; because oxygen is required, cellular respiration is aerobic. 3. Cellular respiration usually involves the complete breakdown of glucose into CO2 and H2O. 4. The net equati ...
ribosomal defects in a mutant deficient in the yajl homolog of the
... by acetate, lactate, succinate and ethanol excretion. The yajL mutant has a low adenylate energy charge favouring glycolytic flux, and a high NADH/NAD ratio favouring fermentations over pyruvate dehydrogenase and the Krebs cycle. DNA array analysis showed upregulation of genes involved in glycolytic ...
... by acetate, lactate, succinate and ethanol excretion. The yajL mutant has a low adenylate energy charge favouring glycolytic flux, and a high NADH/NAD ratio favouring fermentations over pyruvate dehydrogenase and the Krebs cycle. DNA array analysis showed upregulation of genes involved in glycolytic ...
Chapter 8 Cellular Respiration 8.1 Cellular Respiration 1. Cellular
... carbohydrates and other metabolites with the resultant buildup of ATP. 2. Cellular respiration consumes oxygen and produces CO2; because oxygen is required, cellular respiration is aerobic. 3. Cellular respiration usually involves the complete breakdown of glucose into CO2 and H2O. 4. The net equati ...
... carbohydrates and other metabolites with the resultant buildup of ATP. 2. Cellular respiration consumes oxygen and produces CO2; because oxygen is required, cellular respiration is aerobic. 3. Cellular respiration usually involves the complete breakdown of glucose into CO2 and H2O. 4. The net equati ...
PPT File
... potential of NADH or FADH2 is converted into phosphoryl-transfer potential of ATP –A useful way to look at electron transport is to consider the change in free energy associated with the movement of electrons from one carrier to another (自由能的改變與電子的移動) •reduction potential (oxidation-reduction potien ...
... potential of NADH or FADH2 is converted into phosphoryl-transfer potential of ATP –A useful way to look at electron transport is to consider the change in free energy associated with the movement of electrons from one carrier to another (自由能的改變與電子的移動) •reduction potential (oxidation-reduction potien ...
cellular-respiration 1
... slowly; therefore ATP is produced gradually. 9. The breakdown of glucose yields synthesis of 38net ATP and 40 total ATP. A. NAD+ and FAD 1. Each metabolic reaction in cellular respiration is catalyzed by a specific enzyme. 2. As a metabolite is oxidized, NAD+ (nicotinamide adenine dinucleotide) acce ...
... slowly; therefore ATP is produced gradually. 9. The breakdown of glucose yields synthesis of 38net ATP and 40 total ATP. A. NAD+ and FAD 1. Each metabolic reaction in cellular respiration is catalyzed by a specific enzyme. 2. As a metabolite is oxidized, NAD+ (nicotinamide adenine dinucleotide) acce ...
Lecture 9: Citric Acid Cycle/Fatty Acid Catabolism
... Radio-label Experiment. The Krebs Cycle was tested by 14C radiolabeling experiments. In 1941, 14C-Acetyl-CoA was used with normal oxaloacetate, labeling only the right side of drawing. But none of the label was released as CO2. Always the left carboxyl group is instead released as CO2, i.e., that fr ...
... Radio-label Experiment. The Krebs Cycle was tested by 14C radiolabeling experiments. In 1941, 14C-Acetyl-CoA was used with normal oxaloacetate, labeling only the right side of drawing. But none of the label was released as CO2. Always the left carboxyl group is instead released as CO2, i.e., that fr ...
File
... 29) A young animal has never had much energy. He is brought to a veterinarian for help and is sent to the animal hospital for some tests. There they discover his mitochondria can use only fatty acids and amino acids for respiration, and his cells produce more lactate than normal. Of the following, w ...
... 29) A young animal has never had much energy. He is brought to a veterinarian for help and is sent to the animal hospital for some tests. There they discover his mitochondria can use only fatty acids and amino acids for respiration, and his cells produce more lactate than normal. Of the following, w ...
(ATP). - WordPress.com
... begins in the small intestine, where bile salts break fat globules into smaller particles called micelles requires enzymes from pancreas to hydrolyze triacylglycerols to yield monoacylglycerols and fatty acids absorbed by intestinal lining ...
... begins in the small intestine, where bile salts break fat globules into smaller particles called micelles requires enzymes from pancreas to hydrolyze triacylglycerols to yield monoacylglycerols and fatty acids absorbed by intestinal lining ...
Chapter 9 - Bulldogbiology.com
... 1. In general terms, distinguish between fermentation and cellular respiration. 2. Write the summary equation for cellular respiration. Write the specific chemical equation for the degradation of glucose. 3. Define oxidation and reduction. 4. Explain in general terms how redox reactions are involved ...
... 1. In general terms, distinguish between fermentation and cellular respiration. 2. Write the summary equation for cellular respiration. Write the specific chemical equation for the degradation of glucose. 3. Define oxidation and reduction. 4. Explain in general terms how redox reactions are involved ...
Photosynthesis
... 1) Light hits antenna pigments of PSII, which passes energy to chlorophyll a, exciting some of its electrons; it gets replacement electrons from H20 molecules, leaving O2 and H+ ions in the lumen ...
... 1) Light hits antenna pigments of PSII, which passes energy to chlorophyll a, exciting some of its electrons; it gets replacement electrons from H20 molecules, leaving O2 and H+ ions in the lumen ...
Chapter 6 notes
... 6.9 The citric acid cycle completes the oxidation of organic molecules, generating many NADH and FADH2 molecules • The citric acid cycle • is also called the Krebs cycle (after the GermanBritish researcher Hans Krebs, who worked out much of this pathway in the 1930s), • completes the oxidation of o ...
... 6.9 The citric acid cycle completes the oxidation of organic molecules, generating many NADH and FADH2 molecules • The citric acid cycle • is also called the Krebs cycle (after the GermanBritish researcher Hans Krebs, who worked out much of this pathway in the 1930s), • completes the oxidation of o ...
answer - RogueBCHES.com
... b) What amino acid and functional group in the esterase site of acetylcholine esterase reacts with the substrate? c) The most common amino acid used by enzymes to carry out ____________ is histidine. d) The (vitamin) ________________ is required to synthesize coenzyme NAD+ for use in metabolic redox ...
... b) What amino acid and functional group in the esterase site of acetylcholine esterase reacts with the substrate? c) The most common amino acid used by enzymes to carry out ____________ is histidine. d) The (vitamin) ________________ is required to synthesize coenzyme NAD+ for use in metabolic redox ...
Paper - Revision Science
... In addition to activity measurements of individual enzymes, analysis of mitochondrial respiration and ATP production rates are performed. This includes the measurement of mitochondrial oxygen consumption in the presence of different substrates, such as pyruvate and α-ketoglutarate. Analysis may s ...
... In addition to activity measurements of individual enzymes, analysis of mitochondrial respiration and ATP production rates are performed. This includes the measurement of mitochondrial oxygen consumption in the presence of different substrates, such as pyruvate and α-ketoglutarate. Analysis may s ...
Physiology Lecture Outline: Enzymes
... CO2? (decreases); HCO3-? (increases); H2O? (decreases; but in the body, is present in such excess that this is not significant) The interaction of enzymes with modulators can both cause diseases and be used to treat them. Many of the substances we call poisons such as cyanide, mercury, arsenic, and ...
... CO2? (decreases); HCO3-? (increases); H2O? (decreases; but in the body, is present in such excess that this is not significant) The interaction of enzymes with modulators can both cause diseases and be used to treat them. Many of the substances we call poisons such as cyanide, mercury, arsenic, and ...
2 - ATP
... Cellular Respiration • A catabolic, exergonic, oxygen (O2) requiring process that uses energy extracted from macromolecules (glucose) to produce energy (ATP) and water (H2O). ...
... Cellular Respiration • A catabolic, exergonic, oxygen (O2) requiring process that uses energy extracted from macromolecules (glucose) to produce energy (ATP) and water (H2O). ...
Course Notes
... At this stage in your biology career, it is important to recognize the concept of BIOCHEMICAL INDIVIDUALITY. The uniqueness of each individual can be attributed to the combination of genetic and epigenetic factors that influence the a person’s metabolism. ...
... At this stage in your biology career, it is important to recognize the concept of BIOCHEMICAL INDIVIDUALITY. The uniqueness of each individual can be attributed to the combination of genetic and epigenetic factors that influence the a person’s metabolism. ...
Bio 6 – Fermentation & Cellular Respiration Lab INTRODUCTION
... and inorganic phosphate (Pi) is exergonic and thus releases energy which cells can use to do any number of things. Once hydrolyzed, ATP can be regenerated from ADP and Pi, though this is endergonic and thus requires energy. The energy needed to regenerate ATP is obtained from “food”, whatever that m ...
... and inorganic phosphate (Pi) is exergonic and thus releases energy which cells can use to do any number of things. Once hydrolyzed, ATP can be regenerated from ADP and Pi, though this is endergonic and thus requires energy. The energy needed to regenerate ATP is obtained from “food”, whatever that m ...
Part 2
... 2. One C is broken off (CO2) and NAD accepts energy (NADH) 3. The second C is broken off (CO2) and NAD accepts the energy…at this point the acetyl group has been split!! 4. The C4 molecules is rearranged, regenerating the oxaloacetate; releasing energy that is stored in ATP, FADH, and NADH. 5. In su ...
... 2. One C is broken off (CO2) and NAD accepts energy (NADH) 3. The second C is broken off (CO2) and NAD accepts the energy…at this point the acetyl group has been split!! 4. The C4 molecules is rearranged, regenerating the oxaloacetate; releasing energy that is stored in ATP, FADH, and NADH. 5. In su ...
Document
... reaction pyruvate loses a CO2 and a hydrogen to form a 2-carbon acetyl compound, which is temporarily attached to another coenzyme called coenzyme A (or just coA), so the product is called acetyl coA. The CO2 diffuses through the mitochondrial and cell membranes by lipid diffusion, out into the tis ...
... reaction pyruvate loses a CO2 and a hydrogen to form a 2-carbon acetyl compound, which is temporarily attached to another coenzyme called coenzyme A (or just coA), so the product is called acetyl coA. The CO2 diffuses through the mitochondrial and cell membranes by lipid diffusion, out into the tis ...
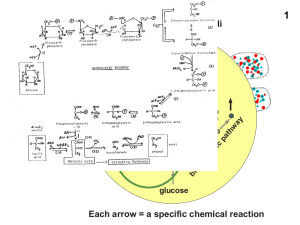
glycolysis and respiration
... h ATP synthase th andd th thus do d nott contribute to ATP production. 22. The proton gradient is used for other things besides ATP synthesis. It is used to pump pyruvate from the cytoplasm into the mitochondrial matrix. The actual yield of ATP is about 26 per glucose. y (7.3 ( x 26 / 720)) x 100 = ...
... h ATP synthase th andd th thus do d nott contribute to ATP production. 22. The proton gradient is used for other things besides ATP synthesis. It is used to pump pyruvate from the cytoplasm into the mitochondrial matrix. The actual yield of ATP is about 26 per glucose. y (7.3 ( x 26 / 720)) x 100 = ...
Citrate Cycle
... This coupled redox reaction directly links the citrate cycle to the electron transport system through the redox conjugate pair FAD/FADH2 which is covalently linked to the enzyme succinate dehydrogenase, an inner mitochondrial membrane protein. Oxidation of succinate results in the transfer of an ele ...
... This coupled redox reaction directly links the citrate cycle to the electron transport system through the redox conjugate pair FAD/FADH2 which is covalently linked to the enzyme succinate dehydrogenase, an inner mitochondrial membrane protein. Oxidation of succinate results in the transfer of an ele ...
Document
... If coupled directly to ADP ATP (7 kcal cost), 46 kcal/mole waste, and heat So the electrons on NADH (and FADH2) are not passed directly to oxygen, but to intermediate ...
... If coupled directly to ADP ATP (7 kcal cost), 46 kcal/mole waste, and heat So the electrons on NADH (and FADH2) are not passed directly to oxygen, but to intermediate ...
ppt
... 2. One C is broken off (CO2) and NAD accepts energy (NADH) 3. The second C is broken off (CO2) and NAD accepts the energy…at this point the acetyl group has been split!! 4. The C4 molecules is rearranged, regenerating the oxaloacetate; releasing energy that is stored in ATP, FADH, and NADH. 5. In su ...
... 2. One C is broken off (CO2) and NAD accepts energy (NADH) 3. The second C is broken off (CO2) and NAD accepts the energy…at this point the acetyl group has been split!! 4. The C4 molecules is rearranged, regenerating the oxaloacetate; releasing energy that is stored in ATP, FADH, and NADH. 5. In su ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.