Glycolysis and Gluconeogenesis
... oxidation and cleavage of glucose ATP generation (with and without oxygen) all cells in the cytosol (the reducing equivalents are transferred to the electron-transport chain by the shuttle) ...
... oxidation and cleavage of glucose ATP generation (with and without oxygen) all cells in the cytosol (the reducing equivalents are transferred to the electron-transport chain by the shuttle) ...
enzyme
... Performing enzymatic transformations in organic solvents renders them more compatible with organic synthesis, this is important because the majority off compounds off interest to organic chemists are insoluble in water. ...
... Performing enzymatic transformations in organic solvents renders them more compatible with organic synthesis, this is important because the majority off compounds off interest to organic chemists are insoluble in water. ...
Lecture 2 * The Kinetics of Enzyme Catalyzed
... – noncompetitive inhibitors are molecules that bind to some other site on the enzyme reducing its catalytic power. • pH. The conformation of a protein is influenced by pH and as enzyme activity is crucially dependent on its conformation, its activity is likewise affected. ...
... – noncompetitive inhibitors are molecules that bind to some other site on the enzyme reducing its catalytic power. • pH. The conformation of a protein is influenced by pH and as enzyme activity is crucially dependent on its conformation, its activity is likewise affected. ...
8.07 Fatty Acid Biosynthesis And Oxidation
... Claisen condensation reactions are performed by enzymes that are members of the thiolase superfamily based on a three-dimensional fold first characterized in a degradative thiolase from Saccharomyces cerevisiae.1,2 These enzymes primarily form dimers, with each subunit sharing a common superfamily t ...
... Claisen condensation reactions are performed by enzymes that are members of the thiolase superfamily based on a three-dimensional fold first characterized in a degradative thiolase from Saccharomyces cerevisiae.1,2 These enzymes primarily form dimers, with each subunit sharing a common superfamily t ...
Enzymes
... • Catalysts speed up chemical reactions. • Enzymes are catalysts in living things. • Catalysts and enzymes work by lowering the activation energy necessary for a reaction to ...
... • Catalysts speed up chemical reactions. • Enzymes are catalysts in living things. • Catalysts and enzymes work by lowering the activation energy necessary for a reaction to ...
Cellular Respiration
... – 2 molecules NADH are created • Important because NADH are Hydrogen ion/proton and e- carriers ...
... – 2 molecules NADH are created • Important because NADH are Hydrogen ion/proton and e- carriers ...
Glycolysis and Anaerobic Respiration Lecture Notes
... cells of yeasts and some microorganisms. In this process, a CO2 molecule is removed from each pyruvic acid molecule and released as a gas. Two hydrogen atoms provided by NADH and H+ ions are transferred to the two carbon molecule converting it to ethyl alcohol. The NADH is oxidized (loses an electro ...
... cells of yeasts and some microorganisms. In this process, a CO2 molecule is removed from each pyruvic acid molecule and released as a gas. Two hydrogen atoms provided by NADH and H+ ions are transferred to the two carbon molecule converting it to ethyl alcohol. The NADH is oxidized (loses an electro ...
Bio AP chp 9 notes
... bound with prosthetic groups that can alternate between reduced and oxidized states as they accept and donate electrons. ...
... bound with prosthetic groups that can alternate between reduced and oxidized states as they accept and donate electrons. ...
1495/Chapter 03
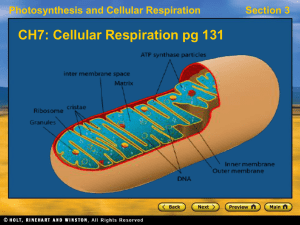
... as shown in Figure 3.7. The inner membrane folds as shelf-like cristae and contains the matrix, an enzyme-rich fluid. The cristae and the matrix are the sites where ATP synthesis occurs. More mitochondria are found in cells that require more energy, such as muscle and liver cells. The two mitochondr ...
... as shown in Figure 3.7. The inner membrane folds as shelf-like cristae and contains the matrix, an enzyme-rich fluid. The cristae and the matrix are the sites where ATP synthesis occurs. More mitochondria are found in cells that require more energy, such as muscle and liver cells. The two mitochondr ...
Cellular Respiration
... A) Most of the free energy available from the oxidation of glucose is used in the production of ATP in glycolysis. B) Glycolysis is a very inefficient reaction, with much of the energy of glucose released as heat. ...
... A) Most of the free energy available from the oxidation of glucose is used in the production of ATP in glycolysis. B) Glycolysis is a very inefficient reaction, with much of the energy of glucose released as heat. ...
Pyruvic acid is chemically groomed for the Krebs cycle
... CO2 Figure 6.10 Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings ...
... CO2 Figure 6.10 Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings ...
Chemiosmotic theory of oxidative phosphorylation. Inhibitors
... (1) Oxidative decarboxilation of pyruvate to acetyl CoA (2) Aerobic oxidation of acetyl CoA by the citric acid cycle (3) Oxidation of fatty acids and amino acids ...
... (1) Oxidative decarboxilation of pyruvate to acetyl CoA (2) Aerobic oxidation of acetyl CoA by the citric acid cycle (3) Oxidation of fatty acids and amino acids ...
Allosteric enzymes
... lipase, are present in the pancreas in their active forms. Presumably, these enzymes would not cause pancreatic cellular damage if released into the pancreatic cell/tissue because there is no starch, glycogen or triglyceride substrate for these enzymes in pancreatic tissue. ...
... lipase, are present in the pancreas in their active forms. Presumably, these enzymes would not cause pancreatic cellular damage if released into the pancreatic cell/tissue because there is no starch, glycogen or triglyceride substrate for these enzymes in pancreatic tissue. ...
Yu-GO
... • between –0.59 and 0.80 (median 0.03) for ‘axial budding’ (21 genes), • and between –0.18 and 0.43 (median 0.19) for ‘NAD biosynthesis’ (6 genes). • The correlations are much higher for terms involving translation mechanism, e.g. from –0.16 to 0.85 (median 0.53) for ‘ribosomal large subunit biogene ...
... • between –0.59 and 0.80 (median 0.03) for ‘axial budding’ (21 genes), • and between –0.18 and 0.43 (median 0.19) for ‘NAD biosynthesis’ (6 genes). • The correlations are much higher for terms involving translation mechanism, e.g. from –0.16 to 0.85 (median 0.53) for ‘ribosomal large subunit biogene ...
I) Choose the best answer: 1- Which of the following metabolites can
... Student number:…………………..Time : 10 min ـــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــ ...
... Student number:…………………..Time : 10 min ـــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــ ...
as Powerpoint presentation
... This process is similar in mitochondria and in many bacteria. Phosphorylation of ADP to ATP is catalysed by a membrane-bound ATP synthase (also called ATPase). The energy for this is provided by the oxidation of NADH2 by oxygen, catalysed by an electron transport chain. This sequence of redox reacti ...
... This process is similar in mitochondria and in many bacteria. Phosphorylation of ADP to ATP is catalysed by a membrane-bound ATP synthase (also called ATPase). The energy for this is provided by the oxidation of NADH2 by oxygen, catalysed by an electron transport chain. This sequence of redox reacti ...
7 energy for cells
... c. How many ATP are produced per glucose molecule as a direct result of the citric acid cycle? _____________ d. What coenzymes carry out oxidation of substrates in the citric acid cycle? ______________ e. Considering your answers to these questions, what are the outputs of the citric acid cycle? ___ ...
... c. How many ATP are produced per glucose molecule as a direct result of the citric acid cycle? _____________ d. What coenzymes carry out oxidation of substrates in the citric acid cycle? ______________ e. Considering your answers to these questions, what are the outputs of the citric acid cycle? ___ ...
Carbohydrate Metabolism
... however, it can be used to produce energy (4 or 6 ATP) by respiratory chain phosphorylation in the mitochondria. 2. This can be done by using special carriers for hydrogen of NADH+H+ These carriers are either dihydroxyacetone phosphate (Glycerophosphate ...
... however, it can be used to produce energy (4 or 6 ATP) by respiratory chain phosphorylation in the mitochondria. 2. This can be done by using special carriers for hydrogen of NADH+H+ These carriers are either dihydroxyacetone phosphate (Glycerophosphate ...
Fermentation of sugars and fermentative enzymes
... experimentally determined degrees of poisoning. Without going into these, I will simply state that on this basis we now understand how quantities of one millionth of a gram and less of a poison may be sufficient to paralyse or annihilate an organism; the quantity of the catalysts required to be inac ...
... experimentally determined degrees of poisoning. Without going into these, I will simply state that on this basis we now understand how quantities of one millionth of a gram and less of a poison may be sufficient to paralyse or annihilate an organism; the quantity of the catalysts required to be inac ...
PDF
... according to the above-described alternation of the two systems. The changes of the above-mentioned enzyme systems affect the energy level in the pupa. Both the amount of the fraction ATP + ADP as of AMP varies along a U-shaped curve. During metamorphosis first a decomposition and later a rebuilding ...
... according to the above-described alternation of the two systems. The changes of the above-mentioned enzyme systems affect the energy level in the pupa. Both the amount of the fraction ATP + ADP as of AMP varies along a U-shaped curve. During metamorphosis first a decomposition and later a rebuilding ...
Enzymes II – How Enzymes Work
... Some enzymes affect substrate molecules by adding charges to the substrate. Such cases often involve acidic or basic R-groups of the enzyme’s amino acids, or metal ions, such as iron or manganese, that are bound to the enzyme. Charge transfer reactions are important in the oxidation-reduction reacti ...
... Some enzymes affect substrate molecules by adding charges to the substrate. Such cases often involve acidic or basic R-groups of the enzyme’s amino acids, or metal ions, such as iron or manganese, that are bound to the enzyme. Charge transfer reactions are important in the oxidation-reduction reacti ...
H - IS MU
... Disease is without any serious consequences. Fructose free diet. Diagnostics: positive reduction test with urine negativ result of specific test for glcose ...
... Disease is without any serious consequences. Fructose free diet. Diagnostics: positive reduction test with urine negativ result of specific test for glcose ...
ch 9ppt
... cytosol into the mitochondria and introduces it into the citric acid cycle. How the process of chemiosmosis utilizes the electrons from NADH and FADH2 to produce ATP. ...
... cytosol into the mitochondria and introduces it into the citric acid cycle. How the process of chemiosmosis utilizes the electrons from NADH and FADH2 to produce ATP. ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.