Electron Carriers
... Six carbon glucose molecule is broken down into 2 three carbon molecules of pyruvic acid Produces 2 net ATP and 2 NADH ...
... Six carbon glucose molecule is broken down into 2 three carbon molecules of pyruvic acid Produces 2 net ATP and 2 NADH ...
Notes Chapter 7 Cellular Respiration
... In alcoholic fermentation, other enzymes convert pyruvic acid into ethyl alcohol and CO2. Through glycolysis, only about 3.5 percent of the energy available from the oxidation of glucose is transferred to ATP. The anaerobic pathways probably evolved very early in the history of life on Earth. ...
... In alcoholic fermentation, other enzymes convert pyruvic acid into ethyl alcohol and CO2. Through glycolysis, only about 3.5 percent of the energy available from the oxidation of glucose is transferred to ATP. The anaerobic pathways probably evolved very early in the history of life on Earth. ...
Solutions to 7.014 Quiz I
... i) What is the main overall product of the dark reactions of photosynthesis? The overall reaction of photosynthesis is 6CO2+6H2OÆC6H12O6(glucose) +6O2, and the main product is glucose. All enzymatic reactions, including those in glycolysis and the Krebs cycle, are reversible. You decide to study whe ...
... i) What is the main overall product of the dark reactions of photosynthesis? The overall reaction of photosynthesis is 6CO2+6H2OÆC6H12O6(glucose) +6O2, and the main product is glucose. All enzymatic reactions, including those in glycolysis and the Krebs cycle, are reversible. You decide to study whe ...
Oxidative Phosphorylation
... Metabolic map is a map that shows components of a pathways of metabolism It is useful in tracing connections between pathways ...
... Metabolic map is a map that shows components of a pathways of metabolism It is useful in tracing connections between pathways ...
- Circle of Docs
... 39. Glutathione peroxidase is an enzyme in various redox reactions which serves to destroy peroxides and free radicals and requires which mineral as a cofactor? a. Zinc b. Selenium c. Iron d. Chromium ...
... 39. Glutathione peroxidase is an enzyme in various redox reactions which serves to destroy peroxides and free radicals and requires which mineral as a cofactor? a. Zinc b. Selenium c. Iron d. Chromium ...
Cell Respiration SAT II Review
... Oxidation of Pyruvate /Link Reaction • When Oxygen is present, the 2 Pyruvates are translocated to the matrix of the mitochondrion where they are converted into 2 Acetyl CoA (C2). • Each Pyruvate releases CO2 (decarboxylation) to form Acetate. • The Acetate is oxidized and gives electrons and H+ io ...
... Oxidation of Pyruvate /Link Reaction • When Oxygen is present, the 2 Pyruvates are translocated to the matrix of the mitochondrion where they are converted into 2 Acetyl CoA (C2). • Each Pyruvate releases CO2 (decarboxylation) to form Acetate. • The Acetate is oxidized and gives electrons and H+ io ...
Name
... CO2= (1 pyruvate dehydrogenase + 2 TCA) = 3 CO2 Total ATP Produced following electron transport by all of the above mitochondrial reactions:___1+12+2=15ATP 2) Draw a diagram that shows with names or numbers the specific enzymes and pathways that feed electrons from FADH2 into electron transport and ...
... CO2= (1 pyruvate dehydrogenase + 2 TCA) = 3 CO2 Total ATP Produced following electron transport by all of the above mitochondrial reactions:___1+12+2=15ATP 2) Draw a diagram that shows with names or numbers the specific enzymes and pathways that feed electrons from FADH2 into electron transport and ...
Oxygen pulls electrons from sugar
... Cellular respiration is a catabolic pathway fueled by oxidizing organic compounds like sugar ...
... Cellular respiration is a catabolic pathway fueled by oxidizing organic compounds like sugar ...
Midterm Review
... by NADH, forming lactate as an end product, with no release of CO2 • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt • Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce ...
... by NADH, forming lactate as an end product, with no release of CO2 • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt • Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce ...
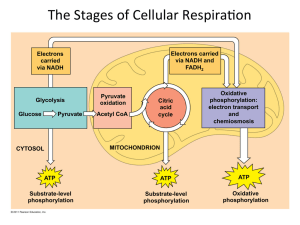
The Stages of Cellular RespiraWon
... (citric acid cycle) Cristae or inner membrane: electron transport chain and oxida3ve phosphoryla3on ...
... (citric acid cycle) Cristae or inner membrane: electron transport chain and oxida3ve phosphoryla3on ...
anaerobic respiration
... When food is broken down, energetic electrons are released. NADH catches the electrons. NADH releases the electrons so that ATP can be made. Metabolism is all of the reactions in the body that involve energy transformation ...
... When food is broken down, energetic electrons are released. NADH catches the electrons. NADH releases the electrons so that ATP can be made. Metabolism is all of the reactions in the body that involve energy transformation ...
Chapter 4 Enzymes and Energy
... prevents it from binding with the substrate. The product may bind with the enzyme at the allosteric site. This is negative feedback. inhibition animation ...
... prevents it from binding with the substrate. The product may bind with the enzyme at the allosteric site. This is negative feedback. inhibition animation ...
Cellular Respiration
... One glucose molecule causes two turns of the Krebs cycle The two turns produce 6 NADH, 2 FADH2, 2 ATP, and 4 CO2. So now there have been 4 molecules of ATP created up to this point (remember the 2 created during glycolysis) ...
... One glucose molecule causes two turns of the Krebs cycle The two turns produce 6 NADH, 2 FADH2, 2 ATP, and 4 CO2. So now there have been 4 molecules of ATP created up to this point (remember the 2 created during glycolysis) ...
CELLULAR RESPIRATION
... • No ATP is generated during ETC; ATP comes from chemiosmosis! • Source of e- = NADH and FADH2 reduction • Source of H+ = same as above! ...
... • No ATP is generated during ETC; ATP comes from chemiosmosis! • Source of e- = NADH and FADH2 reduction • Source of H+ = same as above! ...
Enzymes - part 1
... Functional groups of protein enzymes are involved in acid-base reactions, covalent bond formation, charge-charge interactions BUT they are less suitable for oxid-reduc and group-transfer reactions SO they use COFACTORS (inorganic ions) COFACTORS may be metal ions (Cu2+, Fe3+, Zn2+) trace amounts of ...
... Functional groups of protein enzymes are involved in acid-base reactions, covalent bond formation, charge-charge interactions BUT they are less suitable for oxid-reduc and group-transfer reactions SO they use COFACTORS (inorganic ions) COFACTORS may be metal ions (Cu2+, Fe3+, Zn2+) trace amounts of ...
Chapter 9. Cellular Respiration STAGE 1: Glycolysis
... So what happens if O2 unavailable? ETC backs up nothing to pull electrons down chain NADH & FADH2 can’t unload H ...
... So what happens if O2 unavailable? ETC backs up nothing to pull electrons down chain NADH & FADH2 can’t unload H ...
Cellular Respiration
... ◦ produces up to 32 ATP molecules from each glucose molecule ◦ captures only about 34% of the energy originally stored in glucose ...
... ◦ produces up to 32 ATP molecules from each glucose molecule ◦ captures only about 34% of the energy originally stored in glucose ...
Chapter 9 / Energy-Releasing Pathways and Biosynthesis I
... Differ in their final electron acceptor Cellular respiration produces more ATP Pyruvate is a key juncture in catabolism Glycolysis occurs in nearly all organisms ...
... Differ in their final electron acceptor Cellular respiration produces more ATP Pyruvate is a key juncture in catabolism Glycolysis occurs in nearly all organisms ...
Handout
... Coupling of these reactions is made possible through ATP So… what does he mean by coupling?” energy retrieved from catabolism is stored in ATP and later released to drive anabolic reactions ...
... Coupling of these reactions is made possible through ATP So… what does he mean by coupling?” energy retrieved from catabolism is stored in ATP and later released to drive anabolic reactions ...
Respiratory chain is the most productive pathway to make ATP
... synthesized by another pathway called citric acid cycle (tricarboxylic acid cycle/krebs cycle). The chief purpose of the citric acid cycle is to supply chemical needs of respiratory chain. Citric acid cycle cannot run unless acetyl group is fed into the cycle through an enzyme cofactor, acetyl coenz ...
... synthesized by another pathway called citric acid cycle (tricarboxylic acid cycle/krebs cycle). The chief purpose of the citric acid cycle is to supply chemical needs of respiratory chain. Citric acid cycle cannot run unless acetyl group is fed into the cycle through an enzyme cofactor, acetyl coenz ...
An Introduction to Metabolism by Dr. Ty C.M. Hoffman
... hydrogen ions, H+). The pumping of protons by the electron transport chains establishes a proton gradient, which is a form of potential energy. The proton gradient represents the remaining useful portion o ...
... hydrogen ions, H+). The pumping of protons by the electron transport chains establishes a proton gradient, which is a form of potential energy. The proton gradient represents the remaining useful portion o ...
Citric Acid Cycle: Central Role in Catabolism Entry of Pyruvate into
... dehydrogenase, cofactor TPP. (TPP, the coenzyme form of vitamin B1 facilitates decarboxylation reactions). Pyruvate gets complexed with TPP and looses a CO2 to become an alcohol. • Step 2. The substrate gets transferred from TPP to lipoamide forming a thioester linkage. In the process, the -C–OH is ...
... dehydrogenase, cofactor TPP. (TPP, the coenzyme form of vitamin B1 facilitates decarboxylation reactions). Pyruvate gets complexed with TPP and looses a CO2 to become an alcohol. • Step 2. The substrate gets transferred from TPP to lipoamide forming a thioester linkage. In the process, the -C–OH is ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.