8.1 – Cell Respiration
... The mitochondrion is the organelle in which the rest of cellular respiration ...
... The mitochondrion is the organelle in which the rest of cellular respiration ...
Chapter 7 Harvesting Energy Slides
... 4 Steps of Aerobic respiration 1. Glycolysis 2. Oxidation of pyruvate 3. Kreb’s cycle (aka Citric Acid Cycle) 4. Electron transport chain ...
... 4 Steps of Aerobic respiration 1. Glycolysis 2. Oxidation of pyruvate 3. Kreb’s cycle (aka Citric Acid Cycle) 4. Electron transport chain ...
Chapter 9: Cellular Respiration and Fermentation (Lectures 12 + 13)
... 3.) “Key Points” slide: Carbons donated by acetyl group are ________(oxidized or reduced?) to CO2 4.) What is the energy yield of the citric acid cycle? What type of phosphorylation produces the GTP? ...
... 3.) “Key Points” slide: Carbons donated by acetyl group are ________(oxidized or reduced?) to CO2 4.) What is the energy yield of the citric acid cycle? What type of phosphorylation produces the GTP? ...
lecture notes-metabolism pathways-complete notes
... Metabolism can be subdivided by - Catabolism: The intracellular process of degrading a compound into smaller and simpler products and generating energy. Glucose to CO2, and H2O, protein to amino acids. - Anabolism: the synthesis of more complex compounds and requires energy. Synthesis of small molec ...
... Metabolism can be subdivided by - Catabolism: The intracellular process of degrading a compound into smaller and simpler products and generating energy. Glucose to CO2, and H2O, protein to amino acids. - Anabolism: the synthesis of more complex compounds and requires energy. Synthesis of small molec ...
Document
... • Reconstructions (e.g. Delaye et al. OLEB in press) cannot reach deep enough • The fact that metabolic enzymes are not well conserved does not mean that they were not there! • Scaffolds (pre-RNA, primitive metabolic reactions) may have disappeared without leaving a trace behind!!! • A more syntheti ...
... • Reconstructions (e.g. Delaye et al. OLEB in press) cannot reach deep enough • The fact that metabolic enzymes are not well conserved does not mean that they were not there! • Scaffolds (pre-RNA, primitive metabolic reactions) may have disappeared without leaving a trace behind!!! • A more syntheti ...
cell respiration notes ap - Wesleyan
... Each NADH makes 3 ATP (drops its electrons at top of ETC; hits all 3 proton pumps) Each FADH2 makes 2 ATP (drops its electrons at Q; skips 1st proton pump; so makes less ATP) Electrons passing down ETC provide energy for pumping H + ions into INTERMEMBRANE SPACE Final electron acceptor at end of ETC ...
... Each NADH makes 3 ATP (drops its electrons at top of ETC; hits all 3 proton pumps) Each FADH2 makes 2 ATP (drops its electrons at Q; skips 1st proton pump; so makes less ATP) Electrons passing down ETC provide energy for pumping H + ions into INTERMEMBRANE SPACE Final electron acceptor at end of ETC ...
Respirometer & Anaerobic Respiration
... Used to investigate the rate of oxygen uptake during respiration Involves an experimental tube (where respiring organism is found) and a control tube both linked by a manometer (measures pressure of a gas). Tubes are sealed from the atmosphere In the experimental tube the organism to be inve ...
... Used to investigate the rate of oxygen uptake during respiration Involves an experimental tube (where respiring organism is found) and a control tube both linked by a manometer (measures pressure of a gas). Tubes are sealed from the atmosphere In the experimental tube the organism to be inve ...
File
... triphosphate (ATP) by breaking down organic compounds. Both autotrophs and heterotrophy undergo cellular respiration to breakdown organic compounds into simpler molecules to release energy. Some energy is used to make ATP which is then used by the cells to do work. The figure below shows that ...
... triphosphate (ATP) by breaking down organic compounds. Both autotrophs and heterotrophy undergo cellular respiration to breakdown organic compounds into simpler molecules to release energy. Some energy is used to make ATP which is then used by the cells to do work. The figure below shows that ...
Enzymes - Kevan Kruger
... 13.Know how to interpret graphs in this section. Why isn’t the temperature graph symmetrical? 14.Explain the lock and key analogy. 15.Another more recent explanation of enzyme action is the "induced fit" theory. Describe the basics of this theory. 16.What is an active site, and how can it be changed ...
... 13.Know how to interpret graphs in this section. Why isn’t the temperature graph symmetrical? 14.Explain the lock and key analogy. 15.Another more recent explanation of enzyme action is the "induced fit" theory. Describe the basics of this theory. 16.What is an active site, and how can it be changed ...
Pyruvate Dehydrogenase Complex (PDC)
... 2. Transaldolase and transketolase convert excess R5P into glycolytic intermediates when NADPH needs are higher than the need for nucleotide biosynthesis. 3. GAP and F6P can be consumed through glycolysis and oxidative phosphorylation. 4. Can also be used for gluconeogenesis to form G6P 5. 1 molecul ...
... 2. Transaldolase and transketolase convert excess R5P into glycolytic intermediates when NADPH needs are higher than the need for nucleotide biosynthesis. 3. GAP and F6P can be consumed through glycolysis and oxidative phosphorylation. 4. Can also be used for gluconeogenesis to form G6P 5. 1 molecul ...
Why would someone take the vitamin niacin?
... 8. Using figure 9.5, describe why electron transport chains are an advantage to living systems. 9. Draw figure 9.6 illustrating and labeling the three stages of aerobic cellular respiration and their products. 10. Compare and contrast substrate-level phosphorylation and oxidative phosphorylation. Ma ...
... 8. Using figure 9.5, describe why electron transport chains are an advantage to living systems. 9. Draw figure 9.6 illustrating and labeling the three stages of aerobic cellular respiration and their products. 10. Compare and contrast substrate-level phosphorylation and oxidative phosphorylation. Ma ...
Chapter 15 Metabolism: Basic concepts and design Part Ⅰ
... ¤ ATP hydrolysis drives metabolism by shifting the equilibrium of coupling reactions chemical energy coupling agent protein conformation shift, e.g., muscle contraction the conc. of ion or molecule on the outside/inside of a cell, e.g., Na+/K+ pump ...
... ¤ ATP hydrolysis drives metabolism by shifting the equilibrium of coupling reactions chemical energy coupling agent protein conformation shift, e.g., muscle contraction the conc. of ion or molecule on the outside/inside of a cell, e.g., Na+/K+ pump ...
Lecture 17/18 - Aerobic and Anaerobic Metabolism
... 3.) “Key Points” slide: Carbons donated by acetyl group are ________(oxidized or reduced?) to CO2 ...
... 3.) “Key Points” slide: Carbons donated by acetyl group are ________(oxidized or reduced?) to CO2 ...
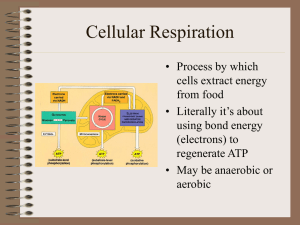
Cellular Respiration
... • 6C glucose split • to 2, 3C pyruvates • Yield 2 ATP • Yield 2 NADH • 10 reaction steps, each catalyzed by specific enzymes. ...
... • 6C glucose split • to 2, 3C pyruvates • Yield 2 ATP • Yield 2 NADH • 10 reaction steps, each catalyzed by specific enzymes. ...
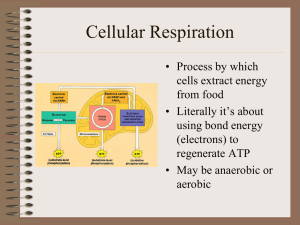
SCI_7726_files/Cellular Respiration
... • 6C glucose split • to 2, 3C pyruvates • Yield 2 ATP • Yield 2 NADH • 10 reaction steps, each catalyzed by specific enzymes. ...
... • 6C glucose split • to 2, 3C pyruvates • Yield 2 ATP • Yield 2 NADH • 10 reaction steps, each catalyzed by specific enzymes. ...
Metabolism without Oxygen
... respiration occurs, then ATP will be produced using the energy of high-energy electrons carried by NADH or FADH2 to the electron transport chain. If aerobic respiration does not occur, NADH must be reoxidized to NAD+ for reuse as an electron carrier for the glycolytic pathway to continue. How is thi ...
... respiration occurs, then ATP will be produced using the energy of high-energy electrons carried by NADH or FADH2 to the electron transport chain. If aerobic respiration does not occur, NADH must be reoxidized to NAD+ for reuse as an electron carrier for the glycolytic pathway to continue. How is thi ...
I. Cellular Energy • ATP: a) When the terminal phosphate is removed
... a) Liver, kidney, & heart cells: shuttle system transfers the electrons from NADH through the inner mitochondrial membrane to an NAD+ in the matrix. These electrons are transferred to the electron transport chain to yield 3 ATP/electron pair (38 ATP’s/glucose molecule). b) Skeletal muscle, brain cel ...
... a) Liver, kidney, & heart cells: shuttle system transfers the electrons from NADH through the inner mitochondrial membrane to an NAD+ in the matrix. These electrons are transferred to the electron transport chain to yield 3 ATP/electron pair (38 ATP’s/glucose molecule). b) Skeletal muscle, brain cel ...
Bis2A 07.2 Fermentation
... In glycolysis, NAD+ is converted to NADH; what happens to the NADH produced? During glycolysis NAD+ is reduced to NADH and glucose is oxidized to pyruvate. During this process the cells must regenerate NAD+ by a second redox reaction. In respiration, this occurs when NADH is used ...
... In glycolysis, NAD+ is converted to NADH; what happens to the NADH produced? During glycolysis NAD+ is reduced to NADH and glucose is oxidized to pyruvate. During this process the cells must regenerate NAD+ by a second redox reaction. In respiration, this occurs when NADH is used ...
Respiration - WordPress.com
... NADH and FADH2 are Oxidised to form NAD+ and FAD. The Hydrogen molecules split into H+ Ions and Electrons (e-). The e- travel along the electron transport chain consisting of Three Electron Carriers within the Inner Mitochondrial Membrane. The e- transfer energy to carriers, causing them to pump H+ ...
... NADH and FADH2 are Oxidised to form NAD+ and FAD. The Hydrogen molecules split into H+ Ions and Electrons (e-). The e- travel along the electron transport chain consisting of Three Electron Carriers within the Inner Mitochondrial Membrane. The e- transfer energy to carriers, causing them to pump H+ ...
08_Cellular respiration ppt
... Glycolysis is the breakdown of glucose into two molecules of pyruvate ...
... Glycolysis is the breakdown of glucose into two molecules of pyruvate ...
CHAPTER OUTLINE
... Cellular respiration is the release of energy from molecules such as glucose accompanied by the use of this energy to synthesize ATP molecules. NAD+ and FAD Cellular respiration involves many individual reactions catalyzed by the coenzymes Nicotinamide adenine dinucleotide (NAD+) and flavin adenine ...
... Cellular respiration is the release of energy from molecules such as glucose accompanied by the use of this energy to synthesize ATP molecules. NAD+ and FAD Cellular respiration involves many individual reactions catalyzed by the coenzymes Nicotinamide adenine dinucleotide (NAD+) and flavin adenine ...
Respiration involves the oxidation of glucose and other compounds
... oxidation and phosphorylation. The uncouplers overcome respiratory control, but do not stop oxidation/the electron transport chain. The energy released from the oxidation of NADH/FADH2 is dissipated as heat. An uncoupler called thermogenin occurs naturally in brown-fat tissue and functions to uncoup ...
... oxidation and phosphorylation. The uncouplers overcome respiratory control, but do not stop oxidation/the electron transport chain. The energy released from the oxidation of NADH/FADH2 is dissipated as heat. An uncoupler called thermogenin occurs naturally in brown-fat tissue and functions to uncoup ...
biology 110
... 4. What is phosporylation. What happens to the store of energy within a molecule when it phosphorylated? 5. What is an electron transport system? 6. Write out the formula for photosynthesis. Be sure to show how many molecules of each reactant and product are used or produced. 7. In question #6, whic ...
... 4. What is phosporylation. What happens to the store of energy within a molecule when it phosphorylated? 5. What is an electron transport system? 6. Write out the formula for photosynthesis. Be sure to show how many molecules of each reactant and product are used or produced. 7. In question #6, whic ...
3.2.1 enzymes - Haiku Learning : Login
... chemical reactions in your body. • Without enzymes, reactions would be too slow to keep you alive. • Catabolic enzymes: enzymes break down large molecules into smaller ...
... chemical reactions in your body. • Without enzymes, reactions would be too slow to keep you alive. • Catabolic enzymes: enzymes break down large molecules into smaller ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.