Microbial Metabolism PowerPoint
... c) redox energy is used to pump H+ into the cell i) creates a higher concentration in ICF d) H+ is moved out through ATPsynthase creating ATP as it moves out e) each NADH has enough energy to produce 3 ATP and each FADH2 can produce 2 i) 30 ATP from NADH and 4 from FADH2 ...
... c) redox energy is used to pump H+ into the cell i) creates a higher concentration in ICF d) H+ is moved out through ATPsynthase creating ATP as it moves out e) each NADH has enough energy to produce 3 ATP and each FADH2 can produce 2 i) 30 ATP from NADH and 4 from FADH2 ...
Electron Transfer Chain
... NADH + H+ + FMN NAD+ + FMNH2 FMNH2 + (Fe-S)ox FMNH· + (Fe-S)red + H+ After Fe-S is reoxidized by transfer of the electron to the next iron-sulfur center in the pathway: FMNH· + (Fe-S)ox FMN + (Fe-S)red + H+ Electrons pass through a series of iron-sulfur centers, and are eventually transferred ...
... NADH + H+ + FMN NAD+ + FMNH2 FMNH2 + (Fe-S)ox FMNH· + (Fe-S)red + H+ After Fe-S is reoxidized by transfer of the electron to the next iron-sulfur center in the pathway: FMNH· + (Fe-S)ox FMN + (Fe-S)red + H+ Electrons pass through a series of iron-sulfur centers, and are eventually transferred ...
Sample pages 1 PDF
... the mitochondrial matrix and the intermembrane space, which, requires ejecting of hydrogen ions into the inter-membrane space in the course of electron transportation for synthesis of water. The structures responsible for transporting ions must do so in a predetermined direction and maintain functio ...
... the mitochondrial matrix and the intermembrane space, which, requires ejecting of hydrogen ions into the inter-membrane space in the course of electron transportation for synthesis of water. The structures responsible for transporting ions must do so in a predetermined direction and maintain functio ...
Vitalens
... Preservative: Thiomersal 0.005%. Pharmacological Action Cytochrome C is involved in the oxidative phosphorylation for synthesizing ATP from ADP. Anhydrous Sodium Succinate (intermediate substance) promotes the production of ATP. Adenosine plays essential role in producing energy required for the vit ...
... Preservative: Thiomersal 0.005%. Pharmacological Action Cytochrome C is involved in the oxidative phosphorylation for synthesizing ATP from ADP. Anhydrous Sodium Succinate (intermediate substance) promotes the production of ATP. Adenosine plays essential role in producing energy required for the vit ...
Photosynthesis
... 5) The H+ pumped into the lumen (and H+ removed from stroma by NADP+) form a chemiosmotic gradient, which is used for synthesis of ATP as those H+ ions return to the stroma by way of a special protein in membrane ATP synthase ...
... 5) The H+ pumped into the lumen (and H+ removed from stroma by NADP+) form a chemiosmotic gradient, which is used for synthesis of ATP as those H+ ions return to the stroma by way of a special protein in membrane ATP synthase ...
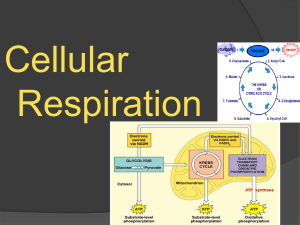
Glycolysis is the major oxidative pathway for glucose
... coenzymes to molecular oxygen via the electron transport chain (ETC); it occurs in the mitochondria. ...
... coenzymes to molecular oxygen via the electron transport chain (ETC); it occurs in the mitochondria. ...
CHAPTER OBJECTIVES Topic 1: Introduction 1. Know the
... 2. Name and describe the four levels of protein structure, indicating the types of forces associated with each level of structure. 3. List the four fundamental bonding interactions found in all proteins. 4. Describe the difference between a structural domain and a subunit. 5. Explain specifically ho ...
... 2. Name and describe the four levels of protein structure, indicating the types of forces associated with each level of structure. 3. List the four fundamental bonding interactions found in all proteins. 4. Describe the difference between a structural domain and a subunit. 5. Explain specifically ho ...
lecture11&12-RS_Major Metabolic Pathways of
... coenzymes to molecular oxygen via the electron transport chain (ETC); it occurs in the mitochondria. ...
... coenzymes to molecular oxygen via the electron transport chain (ETC); it occurs in the mitochondria. ...
ch24a_wcr
... 2 The electrons are transferred from one complex to another in the membrane. Each complex is reduced and then oxidized, releasing energy that is used to pump H+ into the intermembrane space. This creates an electrochemical gradient between the matrix and the intermembrane space. Coenzyme Q (ubiquino ...
... 2 The electrons are transferred from one complex to another in the membrane. Each complex is reduced and then oxidized, releasing energy that is used to pump H+ into the intermembrane space. This creates an electrochemical gradient between the matrix and the intermembrane space. Coenzyme Q (ubiquino ...
Biology 5.3 Cellular Respiration - Chemistry
... lactic acid. For example, during exercise, pyruvate in muscles is converted to lactate when muscles must operate without enough oxygen. ...
... lactic acid. For example, during exercise, pyruvate in muscles is converted to lactate when muscles must operate without enough oxygen. ...
1 Name Chapter 3 Reading Guide Nucleic Acids, Proteins, and
... c. Explain the difference between your answer for the time of (A) and (B). Disulfide bridges are necessary for protein tertiary structure and must form before the enzyme active site can reappear, but there are other chemical interactions, such as hydrogen bonding and hydrophobic interactions, that o ...
... c. Explain the difference between your answer for the time of (A) and (B). Disulfide bridges are necessary for protein tertiary structure and must form before the enzyme active site can reappear, but there are other chemical interactions, such as hydrogen bonding and hydrophobic interactions, that o ...
04. Introduction to metabolism
... blocks. Stage II. Amino acids, fatty acids and glucose are oxidized to common metabolite (acetyl CoA) Stage III. Acetyl CoA is oxidized in citric acid cycle to CO2 and water. As result reduced cofactor, NADH2 and FADH2, are formed which give up their electrons. Electrons are transported via the tiss ...
... blocks. Stage II. Amino acids, fatty acids and glucose are oxidized to common metabolite (acetyl CoA) Stage III. Acetyl CoA is oxidized in citric acid cycle to CO2 and water. As result reduced cofactor, NADH2 and FADH2, are formed which give up their electrons. Electrons are transported via the tiss ...
The nature of matter
... up our DNA, which include carbon, oxygen, hydrogen, nitrogen, and phosphorus atoms. ...
... up our DNA, which include carbon, oxygen, hydrogen, nitrogen, and phosphorus atoms. ...
Role of cryo-ET in membrane bioenergetics research
... densities attributed to PSII in thylakoid membranes (right) and the atomic model of PSII, PDB code 2O01 filtered to 30 Å (1 Å = 0.1 nm) (surface view, left; slice through volume, middle). Both densities are roughly the same size, two-fold symmetric and have two kidney-shaped protrusions on one side o ...
... densities attributed to PSII in thylakoid membranes (right) and the atomic model of PSII, PDB code 2O01 filtered to 30 Å (1 Å = 0.1 nm) (surface view, left; slice through volume, middle). Both densities are roughly the same size, two-fold symmetric and have two kidney-shaped protrusions on one side o ...
Transport in Bacterial Cells
... • A few molecules that are small can pass through the phospholipid bilayer by the process of diffusion • Passive transport does not require an energy input ...
... • A few molecules that are small can pass through the phospholipid bilayer by the process of diffusion • Passive transport does not require an energy input ...
OriginOfLife
... time and time again, just to pump a proton over a membrane. Like saving up to buy something, eventually the proton gradient will be enough to produce one pyrophosphate or one ATP. The upshot is that proton gradients enable cells to grow, to leave the vents. Indeed, it appears Mitchell’s oddity is a ...
... time and time again, just to pump a proton over a membrane. Like saving up to buy something, eventually the proton gradient will be enough to produce one pyrophosphate or one ATP. The upshot is that proton gradients enable cells to grow, to leave the vents. Indeed, it appears Mitchell’s oddity is a ...
What Do Enzymes Do
... simpler building blocks and then use those building blocks to create the new components they require. The breaking down of complex organic molecules occurs via catabolic pathways and usually involves the release of energy. Through catabolic pathways, polymers such as proteins, nucleic acids, and pol ...
... simpler building blocks and then use those building blocks to create the new components they require. The breaking down of complex organic molecules occurs via catabolic pathways and usually involves the release of energy. Through catabolic pathways, polymers such as proteins, nucleic acids, and pol ...
photosynthesis - Northwest ISD Moodle
... using sun’s energy to make ATP using CO2 & water to make sugar in chloroplasts (leaf) allows plants to grow makes a waste product ...
... using sun’s energy to make ATP using CO2 & water to make sugar in chloroplasts (leaf) allows plants to grow makes a waste product ...
Rad24 Interaction with Yeast RPA Table S4. Other novel putative
... Beta subunit of fatty acid synthetase ...
... Beta subunit of fatty acid synthetase ...
C8eBookCh02LegendsTables Ù Figure 2.1 Who tends this garden
... covalent bond consists of a pair of shared electrons. The number of electrons required to complete an atom’s valence shell generally determines how many bonds that atom will form. Four ways of indicating bonds are shown; the spacefilling model comes closest to representing the actual shape of the mo ...
... covalent bond consists of a pair of shared electrons. The number of electrons required to complete an atom’s valence shell generally determines how many bonds that atom will form. Four ways of indicating bonds are shown; the spacefilling model comes closest to representing the actual shape of the mo ...
lecture slides of chap8
... Electron Configurations of Cations of Transition Metals When a cation is formed from an atom of a transition metal, electrons are always removed first from the ns orbital and then from the (n – 1)d orbitals. ...
... Electron Configurations of Cations of Transition Metals When a cation is formed from an atom of a transition metal, electrons are always removed first from the ns orbital and then from the (n – 1)d orbitals. ...
Enzymes and Metabolic Pathways
... the glucose molecule is no longer considered to be glucose when you are looking at the diffusion gradient. So, glucose is always higher outside the cell than inside, and this drives the movement of glucose into the cell. Secondly, once you add the phosphate to the glucose, it is now charged and it c ...
... the glucose molecule is no longer considered to be glucose when you are looking at the diffusion gradient. So, glucose is always higher outside the cell than inside, and this drives the movement of glucose into the cell. Secondly, once you add the phosphate to the glucose, it is now charged and it c ...
Chemical Bonds
... Polymers can be broken down into their component parts through a process called hydrolysis. (lysis= to ...
... Polymers can be broken down into their component parts through a process called hydrolysis. (lysis= to ...
Fermentations
... The balanced net reaction for the pyruvate-ferredoxin oxidoreductase reaction: pyruvate + CoA + 2 FDox + 2 H+ ----- Δ2e- ----> acetyl-CoA + CO2 + 2 FDox + H2 Note that the iron-sulfur protein Ferredoxin (FD) merely shuttles electrons from the substrate to H+, and is regenerated in the process. The P ...
... The balanced net reaction for the pyruvate-ferredoxin oxidoreductase reaction: pyruvate + CoA + 2 FDox + 2 H+ ----- Δ2e- ----> acetyl-CoA + CO2 + 2 FDox + H2 Note that the iron-sulfur protein Ferredoxin (FD) merely shuttles electrons from the substrate to H+, and is regenerated in the process. The P ...
Oxidative phosphorylation
Oxidative phosphorylation (or OXPHOS in short) is the metabolic pathway in which the mitochondria in cells use their structure, enzymes, and energy released by the oxidation of nutrients to reform ATP. Although the many forms of life on earth use a range of different nutrients, ATP is the molecule that supplies energy to metabolism. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis.During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme called ATP synthase; this process is known as chemiosmosis. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor.Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging (senescence). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.