* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The nature of matter
Cell-penetrating peptide wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Expanded genetic code wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Protein adsorption wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
List of types of proteins wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
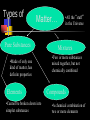
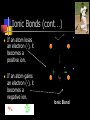
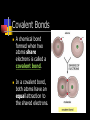
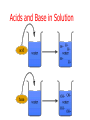
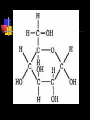
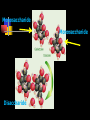
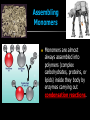
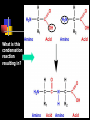
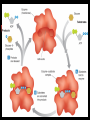
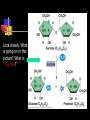
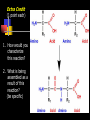
Unit Sections: 1- Atoms, Molecules, and Bonds 2- Water: The Universal Solvent 3- Acids, Bases, and Solutions 4- Organic Macromolecules 5- Polymers and Monomers: Assembly and Disassembly 6- Enzymes What is Matter? -Matter is the “stuff” that makes up everything in the universe. -Matter can be any shape, texture, or color. -Matter has the ability to change states… Types of Matter… Pure Substances •Made of only one kind of matter, has definite properties Elements •Cannot be broken down into simpler substances •All the “stuff” in the Universe Mixtures •Two or more substances mixed together, but not chemically combined Compounds •A chemical combination of two or more elements Particles of matter The smallest possible particle of matter that retains its unique properties is called an atom. Groups of atoms that combine together and act as single units are called molecules. The forces that hold atoms together are called chemical bonds. How small is an atom?……. There are 2,000,000,000,000,000,000,000 atoms of oxygen in one drop of water! (two thousand billion billion)…. Inside an atom An atom is the smallest unit of matter that still retains the properties of a particular element. Atoms and electrons… The nucleus is surrounded by negatively charged electrons which move extremely fast. It is impossible to know where any electron is at any given time… Atoms and Electrons (cont…) Atoms are 99.99% empty space… nucleus Electrons (cont…) Electrons are about 1/2000th the size of protons and neutrons. Proton (+) Electron (-) Ionic Bonds When an atom has fewer than 4 valence electrons, they will often transfer them to another atom that has more than four valence electrons. Ionic Bonds (cont…) An “ion” is an atom that is electrically charged. It has either lost or gained electrons. Ionic Bonds (cont…) If an atom loses an electron (-), it becomes a positive ion. If an atom gains an electron (-), it becomes a negative ion. Ionic Bond Ionic Bonds (cont…) An ionic bond is the attraction between two oppositely charged ions. This results in a neutral compound, commonly known as a “salt.” Ions (cont…) What charge does a chloride ion have? What charge does a magnesium ion have? How many chloride ions would be needed to balance out the charge of a magnesium atom? - 1- (Cl ) 2+ 2+ (Mg ) 2 (MgCl2) Covalent Bonds A chemical bond formed when two atoms share electrons is called a covalent bond. In a covalent bond, both atoms have an equal attraction to the shared electrons. Covalent Bonds The number of bonds an atom will form is dependent on the number of electrons it needs to make a total of 8…. The “octet rule” Polar molecules Polar molecules have a slightly negative charge at one end, and a slightly positive charge at the other end. Attractions between molecules Because of the unequal pull of electrons exhibited by polar molecules. They have a tendency to attract one another. Non-Polar molecules do not attract one another as much, and therefore exhibit different properties. Water: A Unique Compound! The cohesive and adhesive properties water exhibits are products of its polar nature. Water’s high boiling point and relatively low freezing point allow it to remain a liquid in most conditions. Water heats slowly and retains heat effectively, allowing organisms to control temperature and maintain homeostasis. Water: the “Universal” Solvent Because water dissolves so many substances, and is so plentiful on Earth, it is referred to as “the Universal Solvent” Particles in Solution When a solution forms, particles of the solute leave one another, and become surrounded by particles of the solvent. Particles in a solution + Na NaCl Cl Ionic solids (salts) in Solution Particles in a solution When molecular solids (such as sugar) are dissolved in water, the molecules disassociate from one another, but stay in tact. Acids and Base in Solution How to recognize an acid… Acid: In Solution HNO3 - nitric acid H+ + NO3- HCl - hydrochloric acid H+ + Cl- H2SO4 - sulfuric acid 2H+ + SO42- HClO4 - perchloric acid H+ + ClO4- HI - hydroiodic acid H+ + I- How to recognize a base Base: LiOH - lithium hydroxide In Solution… Li+ + OH- NaOH - sodium hydroxide Na+ + OH- KOH - potassium hydroxide K+ + OH- Ca(OH)2 – calcium hydroxide Ca2+ + OH- Ba(OH)2 - barium hydroxide Ba2+ + OH- Properties of Acids Although tasting a compound as a means of identifying it is never smart, you taste acidic compounds every day… SOUR!!! Properties of acids (cont.) Acids are corrosive, meaning they wear away other materials. Some metals react with acids to create hydrogen gas. Properties of Acids (cont.) Litmus paper changes color in the presence of an acid or a base. In the presence of an acid, litmus paper turns red… Everyday acids… Vitamin C is an acid present in fruits. Acid in our stomach helps digest food. Hydrochloric and sulfuric acid are used extensively in industry, as cleaning agents, and in fertilizers. Properties of Bases Bases taste bitter… Bases feel slippery… Properties of Bases cont. Bases turn litmus paper blue Bases do not react with carbonates Everyday Bases Drain cleaners, and various household cleaning products are made with ammonia (NH3) a powerful base… Cement is made using the base Calcium Hydroxide (CaOH2). Bases neutralize excess stomach acid… Measuring pH The Chemistry of Carbon Organic chemistry is the study of all compounds that contain bonds between carbon atoms. Carbon atoms have four valence electrons that can join with the electrons from other atoms to form strong covalent bonds. A carbon atom can bond to other carbon atoms, giving it the ability to form chains that are almost unlimited in length. The Chemistry of Carbon Living organisms are made of molecules that consist of carbon and other elements. Chains of carbon can even close upon themselves to form rings. Carbon has the ability to form millions of different large and complex structures. Copyright Pearson Prentice Hall Life with Carbon Nutrients are substances that provide the energy and raw materials the body needs to grow, repair itself, and function properly. The 4 classes of polymers found in all living things are carbohydrates, proteins, fats, and nucleic acids. Macromolecules Macromolecules Macromolecules are formed by a process known as polymerization. The smaller units, or monomers, join together to form polymers. Carbohydrates Living things use carbohydrates as their main source of energy. Plants and some animals also use carbohydrates for structural purposes. Carbohydrates Carbohydrates are energy rich organic molecules composed of carbon, hydrogen, and oxygen. Complex Carbohydrates Simple Carbohydrates glucose Cellulose, starch Carbohydrates Starches and sugars are examples of carbohydrates that are used by living things as a source of energy. Glucose Monosaccharide Monosaccharide Disaccharide Proteins Most of the body’s structures are composed of proteins. Proteins are complex polymers composed of a specific arrangement of monomers called amino acids. Proteins Some proteins control the rate of reactions and regulate cell processes. Some proteins are used to form bones and muscles. Other proteins transport substances into or out of cells or help to fight disease. Proteins Proteins can have up to four levels of organization: Amino acids have a specific protein chain. The amino acids within a chain can be twisted or folded. The chain itself is folded. If a protein has more than one chain, each chain has a specific arrangement in space. Proteins Amino Acids The instructions for arranging Proteins (cont.) The body uses the proteins we eat to build and repair body parts. Proteins are broken down in the digestive process and the monomers are re-assembled into thousands of proteins. Lipids Like carbs, lipids are energy rich polymers made of carbon, hydrogen, and oxygen. Lipids include fats, oils, waxes, and cholesterol. Fatty acid (saturated) Glycerol Fatty acid (unsaturated) Unsaturated fat Nucleic Acids Nucleic acids (also called nucleotides) are the molecules which make up our DNA (also our RNA). There are only four different kinds of nuclotides that make up our DNA, which include carbon, oxygen, hydrogen, nitrogen, and phosphorus atoms. Nucleic Acids The differences among living things are determined by the order of nucleotides in the DNA. Assembling Monomers Monomers are almost always assembled into polymers (complex carbohydrates, proteins, or lipids) inside they body by enzymes carrying out condensation reactions. What is this condensation reaction resulting in? Disassembling polymers The process of digestion (as well as thousands of other cellular functions) requires that macromolecules be broken down at the molecular level. This process, called depolymerization, requires specialized enzymes to carry out hydrolysis. Enzymes Enzymes are special proteins that can break large molecules into small molecules. Different types of enzymes can break down different nutrients: carbohydrase or amylase enzymes break down starch into sugar. protease enzymes break down proteins into amino acids lipase enzymes break down fats into fatty acids and glycerol. Getting Reactions Started There is always a minimum amount of energy needed to start a chemical reaction…this is referred to as the “activation energy.” Enzymes A catalyst is a substance that speeds up the rate of a chemical reaction. Catalysts work by lowering a reaction's activation energy. Biological catalysts are called enzymes. Enzymes Enzymes play essential roles in: regulating chemical pathways. making material that cells need. releasing energy. transferring information. Enzymes Lowering the activation energy has a dramatic effect on how quickly the reaction is completed. Enzyme Action The Enzyme-Substrate Complex 1 Enzymes provide a site where reactants can be brought together to react, reducing the energy needed for reaction. The reactants of enzyme-catalyzed reactions are known as substrates. Enzyme Action 2 The substrates bind to the active site on the enzyme forming an enzyme-substrate complex. The fit is so precise that the active site and substrates are often compared to a lock and key. Enzyme Action 3 The enzyme and substrates remain bound together until the reaction is done and the substrates are converted to products. The products of the reaction are released and the enzyme is free to start the process again. An Enzyme-Catalyzed Reaction Regulation of Enzyme Activity Enzymes can be affected by any variable that influences a chemical reaction. Enzymes work best at certain pH values. Many enzymes are affected by changes in temperature. Specialized enzymes target specific macromolecules… Look closely. What is going on in this picture? What is “Sucrase?” That’s All Folks! Extra Credit (1 point each) 1. How would you characterize this reaction? 2. What is being assembled as a result of this reaction? (be specific)