the squared modulus of the wave function is the probability density
... The good news is that the Schroedinger equation for the hydrogen atom has an EXACT ANALYTICAL solution! (this is one of the few problems in Quantum Mechanics that does have such a solution – most problems in QM cannot be solved exactly). The bad news, however, is that the procedure of solving the eq ...
... The good news is that the Schroedinger equation for the hydrogen atom has an EXACT ANALYTICAL solution! (this is one of the few problems in Quantum Mechanics that does have such a solution – most problems in QM cannot be solved exactly). The bad news, however, is that the procedure of solving the eq ...
The world of Atoms - University of California, Irvine
... - that is to say, of a theory which represents things themselves and not merely the probability of their occurrence. On the other hand, it seems to me certain that we must give up the idea of complete localization of the particle in a theoretical model. This seems to me the permanent upshot of Heise ...
... - that is to say, of a theory which represents things themselves and not merely the probability of their occurrence. On the other hand, it seems to me certain that we must give up the idea of complete localization of the particle in a theoretical model. This seems to me the permanent upshot of Heise ...
Chapter 31 Quantum Mechanics and Atomic Physics
... hydrogen atom in terms of four quantum numbers: (1) the principal quantum number n, which can have the integer values n = 1, 2, 3, ...; (2) the orbital quantum number l , which can have values l = 0, 1, 2, ..., (n 1); (3) the magnetic quantum number ml, which can have positive and negative integer v ...
... hydrogen atom in terms of four quantum numbers: (1) the principal quantum number n, which can have the integer values n = 1, 2, 3, ...; (2) the orbital quantum number l , which can have values l = 0, 1, 2, ..., (n 1); (3) the magnetic quantum number ml, which can have positive and negative integer v ...
Homework 3
... 1. Explain the terms wavelength and amplitude. If a photon has a frequency of 9 1010 Hz what is its wavelength? Which region of the electromagnetic spectrum does this correspond to? ...
... 1. Explain the terms wavelength and amplitude. If a photon has a frequency of 9 1010 Hz what is its wavelength? Which region of the electromagnetic spectrum does this correspond to? ...
Slide 1
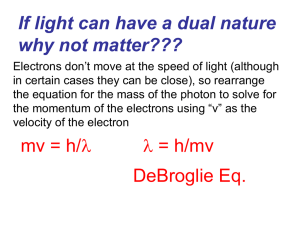
... If light can have a dual nature why not matter??? Electrons don’t move at the speed of light (although in certain cases they can be close), so rearrange the equation for the mass of the photon to solve for the momentum of the electrons using “v” as the velocity of the electron ...
... If light can have a dual nature why not matter??? Electrons don’t move at the speed of light (although in certain cases they can be close), so rearrange the equation for the mass of the photon to solve for the momentum of the electrons using “v” as the velocity of the electron ...
Relativity Problem Set 7 - Solutions Prof. J. Gerton October 24, 2011
... so-called virial theorem, valid also in classical mechanics). So, U α~c α2 me c2 E(= En ) = ...
... so-called virial theorem, valid also in classical mechanics). So, U α~c α2 me c2 E(= En ) = ...
CHEMISTRY 1A
... a. The quantum number, n, describes the _________and _________of an atomic orbital. b. The shape of an atomic orbital is given by the quantum number _________. c. The maximum number of orbitals that may be associated with the following set of quantum numbers n = 5 and l = 3 is _________. d. The maxi ...
... a. The quantum number, n, describes the _________and _________of an atomic orbital. b. The shape of an atomic orbital is given by the quantum number _________. c. The maximum number of orbitals that may be associated with the following set of quantum numbers n = 5 and l = 3 is _________. d. The maxi ...
Problem set 3
... 1. Recall that the angular momentum raising operator is L+ = ~eiφ (∂θ + i cot θ ∂φ ). Use this to find L− . 2. Use the above formulae for L± to find the coordinate representation of the angular momentum basis states Y11 , Y10 and Y1,−1 up to normalization. 3. Write out the 9 equations summarized in ...
... 1. Recall that the angular momentum raising operator is L+ = ~eiφ (∂θ + i cot θ ∂φ ). Use this to find L− . 2. Use the above formulae for L± to find the coordinate representation of the angular momentum basis states Y11 , Y10 and Y1,−1 up to normalization. 3. Write out the 9 equations summarized in ...
Degeneracy of Hydrogen atom
... In quantum mechanics, an energy level is said to be degenerate if it corresponds to two or more different measurable states of a quantum system. Conversely, two or more different states of a quantum mechanical system are said to be degenerate if they give the same value of energy upon measurement. T ...
... In quantum mechanics, an energy level is said to be degenerate if it corresponds to two or more different measurable states of a quantum system. Conversely, two or more different states of a quantum mechanical system are said to be degenerate if they give the same value of energy upon measurement. T ...
CH7 handout is here.
... 8. Heisenberg uncertainty principle states that we cannot know exactly the position and velocity of an electron both at the same instant. Explain what we studied under ‘position’ and under ‘velocity’. What were the assumptions when studying ‘position’? “velocity”? ...
... 8. Heisenberg uncertainty principle states that we cannot know exactly the position and velocity of an electron both at the same instant. Explain what we studied under ‘position’ and under ‘velocity’. What were the assumptions when studying ‘position’? “velocity”? ...
Modern physics
... • Bohr quantized angular momentum, for better H atom model. • Bohr model explained observed H spectra, derived En = E/n2 and phenomenological Rydberg constant • Quantum numbers n, l, ml (Zeeman effect) • Solution to Schrodinger equation showed that En = E/l(l+1) • Pauli proposed spin (ms=1/2), and ...
... • Bohr quantized angular momentum, for better H atom model. • Bohr model explained observed H spectra, derived En = E/n2 and phenomenological Rydberg constant • Quantum numbers n, l, ml (Zeeman effect) • Solution to Schrodinger equation showed that En = E/l(l+1) • Pauli proposed spin (ms=1/2), and ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).