Lec-23_Strachan
... When light is incident on a system of atoms, both stimulated absorption and stimulated emission are equally probable Generally, a net absorption occurs since most atoms are in the ground state If you can cause more atoms to be in excited states, a net emission of photons can result This situation is ...
... When light is incident on a system of atoms, both stimulated absorption and stimulated emission are equally probable Generally, a net absorption occurs since most atoms are in the ground state If you can cause more atoms to be in excited states, a net emission of photons can result This situation is ...
Electron Orbits
... read the literature well enough -- and you know how that happens. On the other hand, one would think that other people would have told us about it. For instance, we had a colloquium at that time in Berlin at which all the important papers were discussed. Nobody discussed Bohr's theory. Why not? The ...
... read the literature well enough -- and you know how that happens. On the other hand, one would think that other people would have told us about it. For instance, we had a colloquium at that time in Berlin at which all the important papers were discussed. Nobody discussed Bohr's theory. Why not? The ...
HW-1-Ch1-Atomic-structure-W16
... 4. Calculate the binding energy per nucleon (MeV) of 56Fe isotope of mass 55.952918 amu. ( P= 1.007277 amu,; N= 1.008665 amu; e- = 5.486 x10-4 amu) ...
... 4. Calculate the binding energy per nucleon (MeV) of 56Fe isotope of mass 55.952918 amu. ( P= 1.007277 amu,; N= 1.008665 amu; e- = 5.486 x10-4 amu) ...
Quantum Mechanics and the Bohr Model - slater science
... •I.D. the source of atomic emission spectra. •Explain how the frequencies of emitted light are related to changes in electron energies. •Distinguish between quantum mechanics and classical mechanics. ...
... •I.D. the source of atomic emission spectra. •Explain how the frequencies of emitted light are related to changes in electron energies. •Distinguish between quantum mechanics and classical mechanics. ...
Quantum Mechanical Model of the Atom
... MODEL OF THE ATOM ESSENTIAL QUESTION: WHAT IS THE CURRENT MODEL OF THE ATOM? ...
... MODEL OF THE ATOM ESSENTIAL QUESTION: WHAT IS THE CURRENT MODEL OF THE ATOM? ...
File
... occupied a small volume at the center of the atom and that most of the volume of the atom was empty space occupied by the electrons. ...
... occupied a small volume at the center of the atom and that most of the volume of the atom was empty space occupied by the electrons. ...
Slide 1
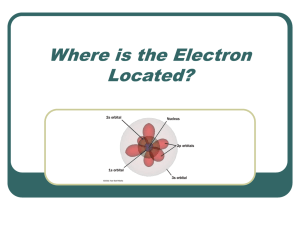
... • PQN–the main E level occupied by the e• n values are positive • As n increases so does the distance from the nucleus • Angular momentum quantum number, l – the shape of the orbital • Values can be 0 and any # lower than what n = (if n=3, l can be 0, 1, and 2) ...
... • PQN–the main E level occupied by the e• n values are positive • As n increases so does the distance from the nucleus • Angular momentum quantum number, l – the shape of the orbital • Values can be 0 and any # lower than what n = (if n=3, l can be 0, 1, and 2) ...
R - University of St Andrews
... All wavelengths are doubled Effect on energy and radii of quantum states? ...
... All wavelengths are doubled Effect on energy and radii of quantum states? ...
Chapt. 5: Quantum Theory of the Hydrogen Atom
... -so, the better we know the position of an object, the worse we know the velocity (p = mv) of the object -not an issue in the macroscopic world, but the limitation is ...
... -so, the better we know the position of an object, the worse we know the velocity (p = mv) of the object -not an issue in the macroscopic world, but the limitation is ...
MIT Physics Graduate General Exams
... Students studying for the Fall 2003 Part I exam found it useful to first categorize each problem by the concept they thought was being tested. Such an approach simplified their subsequent attempt at solving the problem. The following is a list of all the concepts they thought could be used as the ba ...
... Students studying for the Fall 2003 Part I exam found it useful to first categorize each problem by the concept they thought was being tested. Such an approach simplified their subsequent attempt at solving the problem. The following is a list of all the concepts they thought could be used as the ba ...
Quantum numbers
... Quantum numbers When solving Schrödinger's equation for the hydrogen model we find wave functions / orbitals. Each orbital is characterized by a series of numbers called Quantum ...
... Quantum numbers When solving Schrödinger's equation for the hydrogen model we find wave functions / orbitals. Each orbital is characterized by a series of numbers called Quantum ...
Quantum Numbers Practice Problems Name: AP Physics Period: 1
... 2. According to the deBroglie theory, electrons orbiting around the nucleus of an atom are waves (as well as particles). How does our understanding of electron waves change when we give up the idea of a simple orbit for an electron (like the ones that Bohr envisioned)? ...
... 2. According to the deBroglie theory, electrons orbiting around the nucleus of an atom are waves (as well as particles). How does our understanding of electron waves change when we give up the idea of a simple orbit for an electron (like the ones that Bohr envisioned)? ...
Chemistry 354 - Homework Set IV
... combustion of a single molecule of sucrose; the distance from sideline to sideline on a football field; the time required for an electron to make one circuit of the nucleus in the Bohr atom; the mass of a hydrogen atom; the mass of Haystacks Calhoun (erstwhile professional wrestler); the distance be ...
... combustion of a single molecule of sucrose; the distance from sideline to sideline on a football field; the time required for an electron to make one circuit of the nucleus in the Bohr atom; the mass of a hydrogen atom; the mass of Haystacks Calhoun (erstwhile professional wrestler); the distance be ...
Balmer Series
... The spectrum of hydrogen is particularly important in astronomy because most of the Universe is made of hydrogen. Emission or absorption processes in the hydrogen atom give rise to several line series, which are sequences of lines corresponding to electron transitions, each ending or beginning with ...
... The spectrum of hydrogen is particularly important in astronomy because most of the Universe is made of hydrogen. Emission or absorption processes in the hydrogen atom give rise to several line series, which are sequences of lines corresponding to electron transitions, each ending or beginning with ...
Quantum Theory and Electrons as Waves
... De Broglie proposed that electrons could behave like waves. ...
... De Broglie proposed that electrons could behave like waves. ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).