المحاضرة الثانية اساسيات الكم
... where m = mass of electron; v = velocity of electron; r = radius of the orbit; h = the Planck constant. ...
... where m = mass of electron; v = velocity of electron; r = radius of the orbit; h = the Planck constant. ...
science 1 small-group tutorial scheme
... Describe in simple terms the molecular and atomic processes which are involved in the hydrogen emission spectrum experiment. ...
... Describe in simple terms the molecular and atomic processes which are involved in the hydrogen emission spectrum experiment. ...
Rutherford–Bohr model
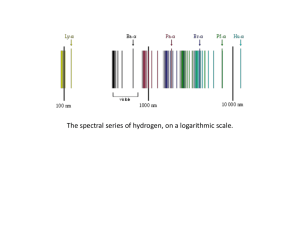
... The diagram to the right illustrates the formation of three series of spectral lines in the atomic emission spectrum of hydrogen. ...
... The diagram to the right illustrates the formation of three series of spectral lines in the atomic emission spectrum of hydrogen. ...
Ch. 6 notes
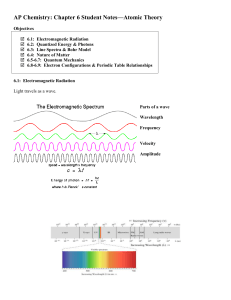
... 6.5-6.7: Quantum Mechanics Developed by Werner Heisenberg (1901-1976), Louis De Broglie (1892-1987), Erwin Schrodinger (1887-1961) This answers the question: Where is the _____________ in the atom? The answer is complex. We can’t say exactly where the atom is. We can only say where we think it _____ ...
... 6.5-6.7: Quantum Mechanics Developed by Werner Heisenberg (1901-1976), Louis De Broglie (1892-1987), Erwin Schrodinger (1887-1961) This answers the question: Where is the _____________ in the atom? The answer is complex. We can’t say exactly where the atom is. We can only say where we think it _____ ...
Slide 1 - s3.amazonaws.com
... Physicists were both mystified and intrigued by Bohr’s theory. They questioned why the energies of hydrogen electron are quantized, or, why is the electron in a Bohr atom restricted or orbiting the nucleus at certain fixed distance? For a decade there is no logical explanation. In 1924, Louis de Bro ...
... Physicists were both mystified and intrigued by Bohr’s theory. They questioned why the energies of hydrogen electron are quantized, or, why is the electron in a Bohr atom restricted or orbiting the nucleus at certain fixed distance? For a decade there is no logical explanation. In 1924, Louis de Bro ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).