* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Macromolecules
Gene expression wikipedia , lookup
Western blot wikipedia , lookup
Point mutation wikipedia , lookup
Peptide synthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Metalloprotein wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Proteolysis wikipedia , lookup
THE MACROMOLECULES OF CELLS
The four macromolecules discussed below are the basis of carrying out the processes of Life. The
compounds all have a skeleton composed of carbon.
Carbon Skeletons and Their Structures
•
Compounds composed of only carbon and hydrogen are known as hydrocarbons
•
The chain of carbon atoms in hydrocarbons is known as carbon skeleton
•
There are various structures of carbon skeletons:
•
single- (Eg. Methane)
•
double unbranched (Eg. Butane)
•
double branched (Eg. Isobutane)
•
double- (Eg. 2-Butene)
•
and triple-bonded compounds (Eg. 3-Butyne)
•
and ring compounds (Eg. Benzene)
Functional Groups in Organic Compounds
•
Functional groups = groups of atoms that participate in chemical reactions.
•
A hydroxy group (OH) is present in organic compounds known as ‘ALCOHOLS’. Example:
sugars contain (OH) group.
•
A carbonyl group (CO) is present in aldehydes
(at the end of a C-skeleton) and ketones
•
(in the middle of a C-skeleton). Example: seen in
sugars.
•
A carboxyl group (COOH) is present in organic acids.
Example: acetic acid, citric acid, in fats, proteins, etc..
•
An amino group (NH2) is present in amines / proteins.
MACROMOLECULES AND MONOMERS
•
There are four main types of macromolecules in living
organisms.
•
The large molecules that compose life are known as
macromolecules.
•
Examples of macromolecules are: carbohydrates,
lipids, proteins and nucleic acids.
•
These macromolecules are called primary metabolites
because they are essential products of metabolism. They are
involved in the growth and development of every plant cell
•
Macromolecules may be composed of several smaller
identical / similar molecular units known as monomers. Such
macromolecules are also called polymers.
•
Cells link monomers together to form a polymer
accompanied by the elimination of water, by a process known as
dehydration synthesis.
Amonomer + Bmonomer --> Dimer + H20
•
Conversely, cells breakdown macromolecules (eg
during digestion) with the help of water. This process is known
as hydrolysis.
Dimer + H20
THE DEHYDRATION REACTION
This type of reaction will join to subunits (monomers) together and remove water in the process.
THE HYDROLYSIS REACTION
A hydrolysis reaction is responsible for breaking down large molecules (eg. digestion). It is just the
opposite of the dehydration reaction.
THIS IMAGE SHOWS BOTH DEHYDRATION AND HYDROLYSIS OF A CARBOHYDRATE.
CARBOHYDRATES
•
Synthesis of carbohydrates begins with carbon fixation that occurs in photosynthesis. Most
carbohydrates are forms by chemical steps that modify glucose.
•
Carbohydrates are made up of monomers known as monosaccharides / single unit sugars.
Example: Glucose and Fructose.
Monosaccharides are represented by the general formula, CH2O. Example: Glucose and Fructose
•
= C6H12O6
•
Glucose and Fructose are isomers, since they have the same chemical formulae. However they
have different structures.
•
In nature, honey is mainly composed of a mixture between glucose and fructose
Disaccharides
•
A carbohydrate composed of two monosaccharides is known as a disaccharide, i.e.
Glucose + Glucose = Maltose
Glucose + Fructose = Sucrose
Glucose + Galactose = Lactose
How Sweet Is Sugar?
•
•
The sweetness of a sugar depends upon how well it can fit into a taste receptor on the tongue.
Sweetness scale is as follows:Natural sweeteners:
Fructose > Glucose > (Maltose = Sucrose) > Lactose
Artificial sweeteners:
Sucralose > Saccharine > Aspartame > Cyclamate
Splenda is Sucralose is 600 times sweeter than sucrose (table
sugar),
Polysaccharides
•
Polysaccharides are long chains of sugar units.
This is the general structure for a polysaccharide. Examples are: Starch, Glycogen, Cellulose and
Chitin.Each of the four differs in some way.
•
Starch is a helically coiled unbranched polymer made up of many units of Glucose. It is a plant
storage sugar.
•
Glycogen is a branched polymer made up of many units of Glucose. It is an animal storage sugar.
•
Cellulose forms structure, it is a fibrous polymer made up of interconnected chains of Glucose
units. It is a major component of cell-walls and of wood.
•
Chitin similar to cellulose, it forms structure in insects and in fungi and is made of interconnected
chains of glucose units. Is the major component of exoskeleton of insects and the cell walls of fungi.
LIPIDS
•
MADE FROM HYDROCARBONS: Hydrocarbons are mainly Hydrogen and carbon. This makes
them insoluble in water. Perhaps you have heard of the following.
•
•
Lipids are mostly non-polar and hydrophobic (water-fearing).
Examples are: fats, phospholipids, waxes, steroids and anabolic steroids.
Notice the long chain hydrocarbon (fatty acid region) which is hydrophobic and the phosphate
group which is hydrophilic .
•
Fats are stored in seeds for use by the germinating embryo.
•
Fats are also called triglycerides because they are composed of three fatty acids linked with one
glycerol.
•
Fats could be saturated or unsaturated.
Fats – Saturated and Unsaturated
Saturated fats are those which have all single bonds and their C- skeletons have
the
maximum number of hydrogen atoms.
Eg. Most animal fats which are solid at room temperature viz. butter, lard, etc.
Unsaturated fats are those that have double bonds and have less than the
maximum number of hydrogen atoms in the carbon skeletons.
Eg. Most plant fats are liquid at room temp. due to the kinks in their structure owing to the
double bonds. Viz. corn oil, olive oil, etc.
Phospholipids, Waxes and Steroids
•
Phospholipids are major components of cell-membranes and have only two fatty acids instead of
three.
•
They have both, a hydrophilic and a hydrophobic component to them.
•
Waxes consist of one fatty acid linked to an alcohol. They are more hydrophobic than fats and
hence can be effective coatings for fruits, insects, animals, etc..
•
Suberin is a hydrophobic compound that is embedded in the Casparian Strip of the Endodermis
of plant roots.
•
Plant Pigment are of two types fat based and hydrophobic as is Chlorophyll amd Carotene and
water based and hydrophilic as is Anthocyanin located in the plant vacuole.
Chlorophyll is the principle light absorbing pigment in photosynthesis. The hydrocarbon tail of both
chlorophyll and carotene allows each to embed in the thylakoid membrane. The light absorbing region of
the chlorophyll has a porphyrin ring with Mg in its center.
•
Steroids are made up of four fused rings, i.e. Cholesterol. This is a component of animal cellmembranes and is used for making other steroids such as sex-hormones. Cholesterol is used in the
manufacture of Bile (important in the digestion of fats) and Estrogen, Progesterone and Testosterone
Anabolic Steroids
Anabolic steroids are synthetic variants of the male sex-hormone, testosterone. They mimic testosterone
in also contributing to body-building.
They have several benefits but also substantial hazards that make a strong case for banning anabolic
steroids.
Benefits and Hazards of Anabolic Steroids
Benefits: treatment of anemia, diseases that destroy body muscle, enhances atheletic performance and
muscle growth, used by athletes, weight-lifters, body-builders and football-players. (This drug is banned by
sports organisations).
Hazards: violent mood-swings, depressions, liver diseases, cancer, cardiovascular problems, reduced sexdrive, breast enlargement, infertility, shrunken testicles, etc.in men.
PROTEINS
•
Proteins are made up of 20 kinds of amino acids in various combinations. Of the 20 a.a.
Humans can not make 8, these are called essential. They must be obtained in the diet. Proper combination
of plants can supply all 20 of the amino acids. One recommended combination is beans (legume) and corn.
The general structure of an amino acid is includes an amino (NH2) and an acid (COOH)] groups.
•
Proteins could be hydrophilic (eg. Serine and Cysteine) or hydrophobic (eg. Leucine).
THIS IS THE BASIC STRUCTURE OF AN AMINO ACID: THE 20 AMINO ACIDS DIFFER BY THE
SIDE CHAINS ('R').
7 Classes of Proteins:
•
There are 7 classes of Proteins, viz.:
•
•
1) Structural proteins (eg. spider silk, hair, tendons, etc..)
2) Contractile proteins (eg. Muscles (actin and myosin))
•
•
•
•
•
3) Storage proteins (eg. Ovalbumin)
4) Defensive proteins (eg. Antibodies)
5) Transport proteins (eg. Hemoglobin)
6) Signal proteins (eg. hormones)
7) Enzyme proteins (eg. digestive enzymes)
Peptide bonds
•
Amino acids are linked together by dehydration synthesis via peptide bonds.
Two amino acids linked together are called a dipeptide.
•
Three amino acids linked together are known as a tripeptide.
•
Several amino acids linked together are known as a polypeptide.
Denaturing of Proteins: Proteins have a specific structure, which is important for their function. If the
structure is distorted or destroyed by heat / ionic concentration / pH change, then the protein is said to be
‘denatured’. Denatured proteins cannot function any more.
Enzymes speed up the rates of Chemical Reactions
● They speed up the rate of chemical reactions by increasing the change of molecular collisions
● They lower the activation energy
● The enzyme is specific for the substrate.
● The substrate binds to the active site of the enzyme and is then converted to products.
● Cofactors and Coenzymes interact with enzymes to increase the efficiency of the rate of the
reaction. Cofactors are small inorganic molecules like Ca, Mg and other ions or can be nonprotein,
organic molecules like vitamins (called coenzymes)
Review the Lab on Enzymes and Germination if you are in the Botany Class
Levels of Protein Structure
1) Primary structure – amino acid sequence.
2) Secondary structure a) polypeptide coiling = alpha helix OR.
b) folding = beta pleated sheets.
(The above secondary structures were discovered by Linus Pauling).
3) Tertiary Structure – overall 3-dimentional shape of a polypeptide.
4) Quaternary Structure – 2 or more 3 D polypeptide chains.
20 amino acids are needed to make all proteins needed for the body. Several (9) are considered
essential, meaning, meaning they are not normally required in the diet, but must be supplied by
the diet. These are the nine amino acids generally regarded as essential for humans:
phenylalanine, valine, threonine, tryptophan, isoleucine, methionine, histidine,
leucine, and lysine.
A vegetarian must combine eating beans and rice to get the 20 amino acids needed in the diet.
NUCLEIC ACIDS
•
Nucleic Acids are made up of monomeric units called ‘nucleotides’.
•
A nucleotide consists of a Nitrogenous base, a sugar and a phosphate.
There are two nucleic acids,. deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).
•
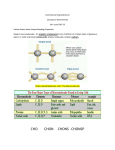
DNA has nitrogenous bases Adenine (A), Guanine (G), Thymine (T) and Cytosine (C).
•
RNA has all the above Nitrogenous bases except for T. In its place, it has Uracil (U).
THESE ARE NITROGENOUS BASES. YOU DON'T NEED TO MEMORIZE THEIR
STRUCTURES. SIMPLY NOTICE THEIR RING LIKE STRUCTURE, THAT THEY CONTAIN
NITROGEN AND THAT THEY EACH DIFFER.
•
•
DNA has a deoxyribose sugar whereas RNA has a ribose sugar.
Both DNA and RNA have Phosphate.
DNA and RNA – Further Comparison
•
DNA is a double stranded double helix whereas RNA is a single stranded polynucleotide strand.
•
DNA has a sugar-phosphate backbone.
•
DNA is present in the nucleus whereas RNA is present in both, the nucleus and the cytoplasm.
•
DNA is transcribed into RNA in the nucleus.
•
RNA is translated into proteins in the ribosomes on the endoplasmic reticulum.
The monomers for these macromolecules are acquired from eating a balanced diet that includes whole
foods, low in calories.
Secondary Metabolism
Plants, unlike animals, have Secondary Metabolism in which they produce phenolics, alkaloids and
terpenoids. Secondary metabolites are non essential for plant growth and development. They provide
protection from environmental stresses, like predation, disease, UV light.
Phenolics are made from the amino acid phenylaanine and tyrosine. They form
●
Lignin which is a component of secondary cell walls. Secondary Metabolism. It is the second
most common organic molecule after cellulose.
● Flavonoids, water soluble molecules common in fruits and vegetables. Tannins are one type,
found in wine
Alkaloids made from several amino acids protect plants from herbivory. Examples include caffeine, heroin,
quinine, nicotine, vinblastine, ephedrine and cocain.
Terpenoids protect plants from herbivores and disease. Examples are phrethrum (an insecticide),
peppermint, latex, terpentines extracted from pines.
Energy
Energy is the ability to do work
There are two types:
Kinetic energy is the energy of motion
Potential energy is stored energy as that found in chemical bonds.
Transformation of energy is known as Thermodynamics.
The two Laws of Thermodynamics:
First : The Low of Conservation of Energy, energy can not be created nor destroyed but converted
from one form to another.
Second: The Law of Entropy. When energy is transformed, Disorder of matter increases in the
universe.
Chemical Reaction are either
Endergonic reactions requires a net input in potential energy
Exergonic reactions that have a net release of free energy.
A chemical reaction can produce a
Oxidation a loss of electrons
Reduction a gain of electrons
Redox reactions couple oxidations with reductions.
Electrons are carried by electron caries like NAFDH, NADPH, FADH2 .