* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapters 16 17 Assig.. - hrsbstaff.ednet.ns.ca
Magnetic monopole wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Electron mobility wikipedia , lookup
Casimir effect wikipedia , lookup
History of electromagnetic theory wikipedia , lookup
Elementary particle wikipedia , lookup
Maxwell's equations wikipedia , lookup
Potential energy wikipedia , lookup
Newton's laws of motion wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Weightlessness wikipedia , lookup
Speed of gravity wikipedia , lookup
Fundamental interaction wikipedia , lookup
Electromagnetism wikipedia , lookup
Anti-gravity wikipedia , lookup
Centripetal force wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Field (physics) wikipedia , lookup
Work (physics) wikipedia , lookup
Lorentz force wikipedia , lookup
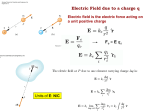
Physics 12 Assignment KEY Chapters 16 & 17 - Electrostatics I. Define the following terms: Inverse square law - the relationship in which the force between two objects is inversely proportional to the square of the distance that separates the centers of the objects; for example, the gravitational and electrostatic forces Electrostatic force - the force between charges at rest Coulomb’s law - the electrostatic force between charges at rest is directly proportional to the magnitudes of the charges and inversely proportional to the square of the distance between their centers Test charge - a charge of small enough magnitude that will not affect the field being measured; it is used to determine the strength of an electric field Electric field intensity - the quotient of the electric force on a unit charge and the magnitude of the charge located at that point; the product of Coulomb’s constant and the charge, divided by the square of the distance from the charge Electric potential difference/ Potential difference (or voltage) - a measure of the amount of electric effort a battery or power supply can expend to push a current through a conductor; calculated as the change in electrical potential energy per unit charge between two locations, such as between two points in a circuit Conductor - an object or type of material which permits the flow of electric charges in one or more directions Insulator - a material whose internal electric charges do not flow freely, and which therefore does not conduct an electric current, under the influence of an electric field Equipotential line – the line (or surface) along which electric potential is constant Electron volt - a unit of energy equal to the work done on an electron in accelerating it through a potential difference of one volt (1 eV = 1.6 x 10-19 J) Chapter 16 II. Questions – Page 496: #1, 2, 4, 6, 8, 9, 15, 18, 19, 20 1. If you charge a pocket comb by rubbing it with a silk scarf, how can you determine if the comb is positively or negatively charged? A plastic ruler is suspended by a thread and then rubbed with a cloth. The rubbing causes the the ruler is negatively charged. Bring the charged comb close to the ruler. If the ruler is repelled by the comb, then the comb is negatively charged. If the ruler is attracted by the comb, then the comb is positively charged. 2. Why does a shirt or blouse taken from a clothes dryer sometimes cling to your body? The clothing gets charged by frictional contact in the tumbling motion of the dryer. The air inside the dryer is dry, and so the clothes can sustain a relatively large static charge. That charged object will then polarize your clothing, and be attracted to you electrostatically. 4. A positively charged rod is brought close to a neutral piece of paper, which it attracts. Draw a diagram showing the separation of charge and explain why attraction occurs. The positively charged rod slightly polarizes the molecules in the paper. The negative charges in the paper are slightly attracted to the part of the paper closest to the rod, while the positive charges in the paper are slightly repelled from the part of the paper closest to the rod. Since the opposite charges are now closer together and the like charges are now farther apart, there is a net attraction between the rod and the paper. 6. Contrast the net charge on a conductor to the “free charges” in the conductor. The net charge on the conductor is the unbalanced charge, or excess charge after neutrality has been established. The net charge is the sum of all of the positive and negative charges in the conductor. If a neutral conductor has extra electrons added to it, then the net charge is negative. If a neutral conductor has electrons removed from it, then the net charge is positive. If a neutral conductor has the same amount of positive and negative charge, then the net charge is zero. Free charges in a conductor refer to those electrons (usually 1 or 2 per atom) that are so loosely attracted to the nucleus that they are “free” to be moved around in the conductor by an external electric force. Neutral conductors have these free electrons. 8. When an electroscope is charged, its two leaves repel each other and remain at an angle. What balances the electric force of repulsion so that the leaves don’t separate further? The electric force of repulsion is balanced by the force of gravity pulling down on the leaves, tending to return them to the vertical position. 9. The form of Coulomb’s law is very similar to that for Newton’s law of universal gravitation. What are the differences between these two laws? Compare also gravitational mass and electric charge. The magnitude of the constant in Newton’s law is very small, while the magnitude of the constant in Coulomb’s law is quite large. Newton’s law says the gravitational force is proportional to the product of the two masses, while Coulomb’s law says the electrical force is proportional to the product of the two charges. Newton’s law only produces attractive forces, since there is only one kind of gravitational mass. Coulomb’s law produces both attractive and repulsive forces, since there are two kinds of electrical charge. 15. When determining an electric field, must we use a positive test charge, or would a negative one do as well? Explain. A negative test charge could be used. For purposes of defining directions, the electric field might then be defined as the OPPOSITE of the force on the test charge, divided by the test charge. 18. Consider the electric field at points A, B, and C in the figure below. First draw an arrow at each point indicating the direction of the net force that a positive test charge would experience if placed at that point, then list the points in order of decreasing field strength (strongest first). At point A, the net force on a positive test charge would be down and to the left, parallel to the nearby electric field lines. At point B, the net force on a positive test charge would be up and to the right, parallel to the nearby electric field lines. At point C, the net force on a positive test charge would be 0. In order of decreasing field strength, the points would be ordered A, B, C. 19. Why can electric field lines never cross? Electric field lines show the direction of the force on a test charge placed at a given location. The electric force has a unique direction at each point. If two field lines cross, it would indicate that the electric force is pointing in two directions at once, which is not possible. 20. Consider a small positive test charge located on an electric field line at some point, such as point P in the figure 16-29a. What is the direction of the velocity and acceleration of the test charge along this line? Discuss. We assume that there are no other forces (like gravity) acting on the test charge. The direction of the electric field line gives the direction of the force on the test charge. The acceleration is always parallel to the force by Newton’s 2nd law, and so the acceleration lies along the field line. If the particle is at rest initially and then released, the initial velocity will also point along the field line, and the particle will start to move along the field line. However, once the particle has a velocity, it will not follow the field line unless the line is straight. The field line gives the direction of the acceleration, or the direction of the change in velocity. III. Problems – Page 497-9: #2, 3, 6, 11, 12, 18,19, 21, 22, 24, 27, 32, 34, 38 2. Two charged smoke particles exert a force of 4.2 x 10-2 N on each other. What will be the force if they are moved so they are only one-eighth as far apart? The magnitude of the Coulomb force is: q q Fe k A 2 B d If we divide the expressions for the two forces, we have: 2 F2 d1 2 F1 d2 F2 (8)2 -2 (4.2 x 10 N) F2 2.7 N 3. Two charged balls are 20.0 cm apart. They are moved and the force on each of them is found to have been tripled. How far apart are they now? The magnitude of the Coulomb force is If we divide the expressions for the two forces, we have which gives r2 = 11.5 cm 6. What is the repulsive electrical force between two protons 5.0 x 10-15 m apart from each other in an atomic nucleus? 1.60 x 10 -19 C 1.60 x 10 -19 C 9 2 2 Fe (9.0 x 10 N m /C ) 9.2 N (5.0 x 10 15 m) 2 11. Particles of charge +70 µC, + 48 µC, and -80 µC are placed in a line, as shown below. The center one is 0.35 m from each of the others. Calculate the net force on the -80 µC charge due to the other two. Let Q1 = +70 µC, Q2 = +48 µC and Q3 = -80 µC. Find the magnitudes of the force on Q3 due to Q1 (F31) and the force on Q3 due to Q2 (F32). Refer to the force vector diagram below. 70 x 10 -6 C - 80 x 10 -6 C 9 2 2 F13 F31 (9.0 x 10 N m /C ) 1.03 x 10 2 N 2 (0.70 m) 48 x 10 -6 C - 80 x 10 -6 C 9 2 2 F23 F32 (9.0 x 10 N m /C ) 2.82 x 10 2 N 2 (0.35 m) Therefore, the net force on Q3 due to the other two charges is: Fnet = (-1.03 x 102 N) + (-2.82 x 102 N) = -3.85 x 102 N (to the right) 12. Three positive particles of equal charge, +11.0 µC, are located at the corners of an equilateral triangle of side 15.0 cm, as shown in the figure below. Calculate the magnitude and direction of the net force on each particle. Because all the charges and their separations are equal, find the magnitude of the individual forces: 11.0 x 10 -6 C 11.0 x 10 -6 C 9 2 2 F1 (9.0 x 10 N m /C ) 48.4 N (0.150 m) 2 The directions of the forces are determined from the signs of the charges and are indicated on the diagram below: For the forces on the top charge, the horizontal force components will cancel. For the net force: Fnet = F1cos30o + F1cos30o + 2F1cos30o Fnet = (48.4 N)cos30o + (48.4 N)cos30o + 2(48.4 N)cos30o Fnet = 83.8 N, up (or away from the center of the triangle) From the symmetry of the triangle, each of the other forces will have the same magnitude and direction away from the center. 18. In one model of the hydrogen atom, the electron revolves in a circular orbit around the proton with a speed of 1.1 x 106 m/s. Determine the radius of the electron’s orbit. The attractive electrostatic force provides the centripetal acceleration of the electron. Note that d = r in the derivation below: Fe Fc k mv 2 r k q A qB r mv 2 (9.0 x 10 9 N m2 /C 2 ) 1.60 x 10 -19 C 1.60 x 10 -19 C r (9.1 x 10 31 kg)(1.1 x 10 6 m/s) 2 q A qB d2 r 2.1 x1 0 10 m 19. A +5.7 µC and a -3.5 µuC charge are placed 25 cm apart. Where can a third charge be placed so that it experiences no net force? If we place a positive charge, it will be repelled by the positive charge and attracted by the negative charge. Thus the third charge must be placed along the line of the charges, but not between them. For the net force to be zero, the magnitudes of the individual forces must be equal: which gives x = 0.91 cm or -0.11 cm. 21. What is the magnitude of the acceleration experienced by an electron in an electric field of 600 N/C? How does the direction of the acceleration depend on the direction of the electric field at that point? How does the direction of the acceleration depend on the electron’s velocity at that point? The acceleration is produced by the force from the electric field: F = qE = ma = (-1.60 x 10-19 C)(+600 N/C) = (9.11 x 10-31 kg)a a = 1.05 x 1014 m/s2 Because the charge on the electron is negative, the direction of force, and thus the acceleration, is opposite to the direction of the electric field. The direction of the acceleration is independent of the velocity. 22. What are the magnitude and direction of the electric force on an electron in a uniform electric field of strength 3500 N/C that points due east? If we take the positive direction to the east, we have: F = qE = (-1.60 x 10-19 C)(+3500 N/C) = -5.6 x 10-16 N (west) 24. A downward force of 8.4 N is exerted on a -8.8 µC charge. What are the magnitude and direction of the electric field at this point? If we take the positive direction up, we have: F = qE E = F/q E = (-8.4 N)/ (-8.8 x 10-6 C) = +9.5 x 105 N/C, up 27. An electron is released from rest in a uniform electric field and accelerates to the north at a rate of 125 m/s2. What is the magnitude and direction of the electric field? The acceleration is produced by the force from the electric field: F = qE = ma = (-1.60 x 10-19 C)(E) = (9.11 x 10-31 kg)( 125 m/s2) E = -7.12 x 10-10 N/C Because the charge on the electron is negative, the direction of force, and thus the acceleration, is opposite to the direction of the electric field, so the electric field is 7.12×10–10 N/C (south). 32. Calculate the electric field at one corner of a square 1.00 m on a side if the other three corners are occupied by 2.80 x 10-6 C charges. The directions of the individual fields are shown in the figure below: We find the magnitudes of the individual fields: Q E1 E 3 k 2 L -6 m2 2.80 x 10 C 9 E1 E 3 (9.0 x 10 N 2 ) C (1.00 m) 2 E1 E 3 2.52 x 10 4 N/C E2 k -6 m2 2.80 x 10 C (9.0 x 10 N 2 ) 1.26 x 10 4 N/C 2 2 C ( 2) m) (L 2) Q 9 Since three E field vectors need to added together to find the net electric field, use vector resolution to find Enet: Vector X-Component (N/C) E1 2.52 x 104 E2 (1.26 x 104) cos45o = 8909.5 E3 0 Enet 34109.55 Y-Component (N/C) 0 (1.26 x 104) sin45o = 8909.5 2.52 x 104 34109.55 E net 34109.55 2 34109.55 2 48238 N/C 4.82 x 10 4 N/C 34109.55 o θ tan -1 45 (from a horizontal) 34109.55 34. Draw, approximately, the electric field lines about two point charges, +Q and -3Q, which are a distance, l, apart. Note: Because the charges are of opposite sign, field lines must radiate out from +q charge and into -3q charge. Because the negative charge has 3 times the charge magnitude as the positive charge, the diagram must show 3 times the amount of field lines coming into the -3q charge as there are radiating out of –q charge. 38. An electron is accelerated in the uniform field E (E = 1.85 x 104 N/C) between two parallel charged plates. The separation of the plates is 1.20 cm. The electron is accelerated from rest near the negative plate and passes through a tiny hole in the positive plate. (a) With what speed does it leave the hole? (b) Show that the gravitational force (i.e. weight) can be ignored. (a) We find the acceleration produced by the electric field: F = qE = ma = (-1.60 x 10-19 C)(1.85 x 104 N/C) = (9.11 x 10-31 kg)a a = 3.24 x 1015 m/s2 Because the field is constant, the acceleration is constant, so we find the speed from vf2 = vi2 + 2ad and since vi = 0 m/s and d = 1.20 x 10-2 m, vf = 8.83 x 106 m/s (b) For the ratio of the two forces, we have mg/qE = (9.11 x 10-31 kg)(9.81 m/s2)/(-1.60 x 10-19 C)(1.85 x 104 N/C) = 3.0 x 10-15 Thus mg << qE Chapter 17 IV. Questions – Page 522: #1, 2, 4, 6 1. If two points are at the same potential, does that mean that no work is done in moving a test charge from one point to the other? Does this imply that no force must be exerted? If two points are at the same potential, then no NET work was done in moving a test charge from one point to the other. Along some segments of the path, some positive work might have been done, but along other segments of the path, negative work would then have been done. And if the object was moved along an equipotential line, then no work would have been done along any segment of the path. Along any segment of the path where positive or negative work was done, a force would have to be exerted. If the object was moved along an equipotential line, then no force would have been exerted along any segment of the path. This is analogous to climbing up and then back down a flight of stairs to get from one point to another point on the same floor of a building. Gravitational potential increased while going up the stairs, and decreased while going down the stairs. A force was required both to go up the stairs and down the stairs. If instead you walked on the level from one point to another, then the gravitational potential was constant, and no force was need to change gravitational potential. 2. Can two equipotential lines cross? Explain. Two equipotential lines cannot cross. That would indicate that a region in space had two different values for the potential. For example, if a 40-V line and a 50-V line crossed, then the potential at the point of crossing would be both 40 V and 50 V, which is impossible. Likewise, the electric field is perpendicular to the equipotential lines. If two lines crossed, the electric field at that point would point in two different directions simultaneously, which is not possible. 4. Is there a point along the line joining two equal positive charges where the electric field is zero? Where the electric potential is zero? Explain. (Refer to diagram to the right): The electric field is zero at the midpoint of the line segment joining the two equal positive charges. The electric field due to each charge is of the same magnitude at that location, because the location is equidistant from both charges, but the two fields are in the opposite direction. Thus the net electric field is zero there. The electric potential is never zero along that line, except at infinity. The electric potential due to each charge is positive, and so the total potential, which is the algebraic sum of the two potentials, is always positive. 6. If a negative charge is initially at rest in an electric field, will it move toward a region of higher potential or lower potential? What about a positive charge? How does the potential energy of the charge change in each case? A negative charge will move toward a region of higher potential. A positive charge will move towards a region of lower potential. The potential energy of each will decrease. V. Problems – Page 522 – 3: #1, 3, 5, 7, 13, 14, 19, 22 1. How much work is needed to move a -8.6 μC charge from ground to a point whose potential is +75V? We find the work done by an external agent from the work-energy theorem: W ΔEk ΔEp q(Vb Va ) (8.6 x 106 C)(75 V 0 V) 6.5 x 10 4 J (done by the field) 3. How much kinetic energy will an electron gain if it falls through a potential difference of 21,000V in a TV picture tube? Because the total energy of the electron is conserved, we have: ΔEk ΔEp 0 ΔEk q(Vb Va ) 0 ΔEk q(Vb Va ) -(-1.60 x 10 -19 C)(21000 V) 3.4 x 10 -15 J 5. How strong is the electric field between two parallel plates 5.2 mm apart if the potential difference between them is 220V? For the uniform electric field between two large, parallel plates, we have: E Vba 220V 4.2 x 104 V/m (N/C) 3 d 5.2 x 10 m 7. What potential difference is needed to give a helium nucleus (Q = 2e) 65.0 keV of E k? Because the total energy of the helium nucleus is conserved, we have: ∆Ek + ∆Ep = 0 ∆Ek + q(VB – VA) = 0 (65.0 x 103 eV) + (+2e)( VB – VA) = 0 which gives, VB – VA = -32.5 x 103 V 13. What is the electric potential 15.0 cm from a 4.00- μC point charge? We find the electric potential of the point charge from: Q V k r 2 6 C 9 Nm 4.00 10 V 9.00 10 2 2 C 15.0 10 m V 2.40 10 5 V 14. A charge Q creates an electric potential of +125 V at a distance of 15 cm. What is the value of Q? Q V k r (125 V)15.0 10 2 m) Q Nm2 9.00 10 9 2 C 9 Q 2.1 10 C 19. Consider point a which is 70 cm north of a -3.8- μC point charge, and point b which is 80 cm west of the charge (as illustrated in the diagram below): Determine (a) Vba = Vb – Va and (b) Eb – Ea (magnitude and direction) (a) We find the electric potentials at the two points. Refer to the diagram below: Q Va k ra Nm2 Va 9.00 10 9 C2 Va - 4.89 10 4 V Q Vb k rb - 3.8 10 6 C 0.70 m Nm2 Vb 9.00 10 9 C2 Vb - 4.28 10 4 V - 3.8 10 6 C 0.80 m Thus the difference is: Vba = Vb – Va = (-4.28 x 104 V) – (-4.89 x 104 V) = +6.1 x 103 V (b) We find the electric fields at the two points. Refer to the electric field diagram below: q Ea k 2 da -6 m2 - 3.8 x 10 C 9 E a (9.0x10 N 2 ) C (0.70 m) 2 N E a 6.98 x 10 4 , toward Q (down) C q Eb k 2 da -6 m2 - 3.8 x 10 C 9 Eb (9.0x10 N 2 ) C (0.80 m) 2 N Eb 5.34 x 10 4 , toward Q (right) C As shown on the vector diagram, the magnitude of Eb – Ea (Eb + -Ea) equals: 2 2 N N Enet 6.98 x 10 4 5.34 x 10 4 8.79 x 10 4 N/C C C 6.98 x 10 4 θ tan -1 4 5.34 x 10 53 o (north of east) 22. In the Bohr model of the hydrogen atom, an electron orbits a proton (the nucleus) in a circular orbit of radius 0.53 x 10-10 m. (a) What is the electric potential at the electron’s orbit due to the proton? (b) What is the kinetic energy of the electron? (c) What is the total energy of the electron in its orbit? (d) What is the ionization energy – that is, the energy required to remove the electron from the atom and take it to r = infinity, at rest? (a) We find the electric potential of the proton from: Q V k r Nm2 1.60 10 19 C Va 9.00 10 9 C 2 0.53 x 10 10 m Va 27.2 V (b) For the electron orbiting the nucleus, the attractive Coulomb force provides the centripetal acceleration: Fe Fc k 1 k 2 r2 qq mv 2 r mv 2 r2 qq 1 mv 2 r 2 qq Ek r kr 1 k 2 qq 1 (27.2 V ) - 1.60 x 10 -19 C E k 2 2.176 x 10 18 J E k 13.6 eV E k (c) The potential energy of the electron is Ep = V/q = (27.2 V)/(-1.60 x 10-19 C) = 4.352 x 10-18 J. The total energy of the electron in its orbit is: ΔEk ΔEp 2.176 x 10 18 J - 4.352 x 10 - 18 J 2.176 x 10 18 J - 13.6 eV (d) Because the final energy of the electron is zero, the ionization energy is 2.176 x 10-18 J or 13.6 eV.