* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CHM 101 - Academic Computer Center
Bent's rule wikipedia , lookup
Click chemistry wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Electrolysis of water wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Bond valence method wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Electrochemistry wikipedia , lookup
Photoelectric effect wikipedia , lookup
Process chemistry wikipedia , lookup
Metallic bonding wikipedia , lookup
Chemical reaction wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Atomic orbital wikipedia , lookup
Stoichiometry wikipedia , lookup
Transition state theory wikipedia , lookup
Microbial metabolism wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Electron scattering wikipedia , lookup
Photoredox catalysis wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
Molecular dynamics wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Hydrogen atom wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Gamma spectroscopy wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Electron configuration wikipedia , lookup
Marcus theory wikipedia , lookup
Electronegativity wikipedia , lookup
Hypervalent molecule wikipedia , lookup
History of molecular theory wikipedia , lookup
Chemical bond wikipedia , lookup
Metalloprotein wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
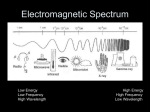
CHM 101 GAGE FALL 2007 NAME ______________________________ DATE ______________________________ EXAM 2 I. Select the best response for each of the following questions or statements. (24) _____ 1. Cold packs, whose temperatures are lowered when ammonium nitrate dissolves in water, are carried by athletic trainers when transporting ice is not possible. Which of the following is true of this reaction? A. H < 0, process is exothermic B. H > 0, process is exothermic C. H < 0, process is endothermic D. H > 0, process is endothermic E. H = 0, since cold packs are sealed _____ 2. Natural gas, or methane, is an important fuel. Combustion of one mole of methane releases 802.3 kilojoules of energy. How much energy does that represent in kilocalories? A. 1.92 10-1 kcal D. 1.92 105 kcal B. 1.92 102 kcal E. 3.36 106 kcal C. 3.36 103 kcal ______ 3. Which scientist first proposed that the electron in the hydrogen atom can have only certain energies? A. B. C. _____ 4. D. E. Rydberg Heisenberg Select the arrangement of electromagnetic radiation which starts with the lowest energy and increases to greatest energy. A. B. C. D. E. _____ 5. Planck Einstein Bohr radio, infrared, ultraviolet, gamma rays radio, ultraviolet, infrared, gamma rays gamma rays, infrared, radio, ultraviolet gamma rays, ultraviolet, infrared, radio infrared, ultraviolet, radio, gamma rays Select the correct electron configuration for Te (Z = 52). A. B. C. [Kr]5s25p64d8 [Kr]5s25d105p4 [Kr]5s24d105p6 [Kr]5s24f14 [Kr]5s24d105p4 D. E. 1 _____ 6. Elements with the highest first ionization energies are found in the ___________ region of the periodic table. A. B. C. _____ 7. P3- > Cl- > K+ > Ca2+ Ca2+ > K+ > Cl- > P3K+ > Cl- > Ca2+ > P3- D. E. K+ > Cl- > P3- > Ca2+ None of these is correct. SrCl2 CsCl ClF D. E. TiF2 S8 Electronegativity is a measure of A. B. C. D. E. _____ 11. low, very negative high, positive or slightly negative low, positive or slightly negative high, very negative None of these is generally correct. In which of these substances are the atoms held together by polar covalent bonding? A. B. C. _____ 10. lower right upper right What is the correct order of decreasing size of the following ions? A. B. C. _____ 9. D. E. Elements with ________________ first ionization energies and ___________ electron affinities generally form cations. A. B. C. D. E. _____ 8. lower left upper left center the energy needed to remove an electron from an atom. the energy released when an electron is added to an atom. the magnitude of the negative charge on an electron. the attraction by an atom for electrons in a chemical bond. the magnitude of the negative charge on a molecule. Arrange oxygen, sulfur, calcium, rubidium and potassium in order of decreasing atomic radius. A. B. C. D. E. O > S > Ca > K > Rb O > S > Ca > Rb > K O > S > Rb > K > Ca O > S > Rb > Ca > K None of these orders is correct. 2 _____ 12. Based on electronegativity trends in the periodic table, predict which of the following compounds will have the greatest % ionic character in its bonds. A. B. C. I I. H2O LiI CaO D. E. RbF HCl Select the best response for each of the following questions or statements. (16) _____ 1. Calculate the number of moles in 17.8 g of the antacid magnesium hydroxide. A. 3.28 mol D. 0.305 mol B. 2.32 mol E. 0.200 mol C. 0.431 mol _____ 2. Calculate the number of oxygen atoms in 29.34 g of sodium sulfate, Na2SO4. A. B. C. _____ 3. 1.244 1023 O atoms 4.976 1023 O atoms 2.409 1024 O atoms D. E. 2.915 1024 O atoms 1.166 1025 O atoms The highly exothermic thermite reaction, in which aluminum reduces iron(III) oxide to elemental iron, has been used by railroad repair crews to weld rails together. 2Al(s) + Fe2O3(s) 2Fe(s) + Al2O3(s) H = -850 kJ What mass of iron is formed when 725 kJ of heat are released? A. B. C. _____ 4. 47 g 65 g 95 g D. E. 112 g 130 g The FM station KDUL broadcasts music at 99.1 MHz. Find the wavelength of these waves. A. B. C. 1.88 10-2 m 0.330 m 3.03 m 5.33 102 m > 103 m D. E. 3 SHOW ALL WORK TO RECEIVE CREDIT FOR PROBLEMS. III. Use the equation below to solve the problems below. 3 LiC2H3O2 + AlCl3 3 LiCl + Al(C2H3O2)3 a. How many grams of lithium chloride can be formed when you react 25.0 grams of lithium acetate with 20.0 g of aluminum chloride? (12) b. If you actually produce 13.6 grams of lithium chloride, what is your % yield? c. A compound is analyzed and found to contain 6.0% lithium, 45.3% chromium, and 48.2% oxygen. Determine the empirical formula. (10) 4 (5) 400 350 300 Energy (kJ) 250 200 150 100 50 0 0 5 10 15 20 25 Reaction Progress IV. Use the graph above to answer the questions below. a. Determine the ΔH for the reaction. _____________________________ (5) b. Is the reaction endothermic or exothermic? Explain your choice. (3) c. On the axes above, sketch what the graph would look like for this reaction if you added a catalyst at the start of the reaction. (2) 5 V. a. Complete the table below with the Lewis dot structure and structural formula with each bond dipole shown on the structural formula. (15) Substance Lewis dot structure Structural formula with bond dipole moments CHFCHF PO33- SCl3H3 b. Oxygen can form either covalent or ionic bonds. Explain the nature of each bond, the conditions under which each forms and the type of substances in each bond. (8) 6