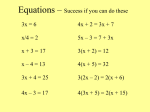* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Equations and Reactions
Photoredox catalysis wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Atomic theory wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Fine chemical wikipedia , lookup
Spinodal decomposition wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical potential wikipedia , lookup
Organic chemistry wikipedia , lookup
Chemical Corps wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Marcus theory wikipedia , lookup
Chemical plant wikipedia , lookup
Safety data sheet wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Drug discovery wikipedia , lookup
Chemical industry wikipedia , lookup
History of chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Process chemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Electrochemistry wikipedia , lookup
Rate equation wikipedia , lookup
Click chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Transition state theory wikipedia , lookup
Chemical reaction wikipedia , lookup
Chemical Equations and Reactions Chapter 8 Notes Section 1: Describing Chemical Reactions Chemical reaction- process by which one or more substances are changed into one or more different substances Reactants- original substances in a chemical reaction Products- resulting substances in a chemical reaction Section 1: Describing Chemical Reactions Chemical equation- uses symbols and formulas to represent identities and relative amounts of the reactants and products in a chemical reaction Example: (NH4)2Cr2O7 N2 + Cr2O3 + 4H20 Reactants Products Indications of a Chemical Reaction Absolute proof of a chemical change can be provided only by chemical analysis of the products. However, certain easily observed changes can indicate that a chemical reaction has taken place: Indications of a Chemical Reaction Evolution of energy as heat and light Heat or light by itself is not necessarily a sign of chemical change, because physical changes can also involve either. Production of a gas when substances are mixed Ex: Bubbles of CO2 form when baking soda and vinegar are mixed Indications of a Chemical Reaction Formation of a precipitate—a solid appears after two solutions are mixed A change in color Characteristics of Chemical Equations Requirements for a properly written chemical equation: The equation must represent known facts that have been identified through chemical analysis in the lab or sources that give results of experiments Characteristics of Chemical Equations The equation must contain correct formulas for the reactants and products *Remember that elements existing primarily as diatomic (H, N, O, and Group 17) are represented by its molecular formula ( H2, N2, O2, etc.). Other elements are represented by atomic symbol (ex: iron, Fe; carbon, C); exceptions are sulfur (S8) and phosphorus (P4). Characteristics of Chemical Equations The law of conservation of mass must be satisfied *Remember that atoms are not created or destroyed in ordinary chemical reactions. The same number of atoms of each element must appear on each side of a correct chemical equation. Add coefficients to balance, if necessary. Characteristics of Chemical Equations Coefficient- small whole number that appears in front of a formula in a chemical equation Placing a coefficient in front of a formula specifies the relative number of moles of the substance; if no coefficient is written, it is assumed to be 1. Characteristics of Chemical Equations Example: (NH4)2Cr2O7 (s) --> N2(g) + Cr2O3(s) + 4H2O(g) The 4 indicates that 4 mol of water are produced for each mole of nitrogen and chromium(III) oxide that is produced. Characteristics of Chemical Equations Word and Formula Equations Word equation- an equation in which the reactants and products in a chemical reaction are represented by words It may help to write a word equation when beginning to write a chemical equation, but it only gives qualitative information; it does not give quantities of the reactants and products. Word and Formula Equations Example: Methane + Oxygen Carbon dioxide + Water Word and Formula Equations The next step is to replace the names of the reactants and products with appropriate symbols and formulas. This creates a formula equation: CH4 + O2 CO2 + H2O Word and Formula Equations Formula equation- qualitatively represents the reactants and products of a chemical reaction by their symbols or formulas Notice that the formula equation does not give information about the amounts of reactants and products. A formula equation meets two of the three requirements for a correct chemical equation; it represents facts and shows correct symbols and formulas. Word and Formula Equations To complete this chemical equation, we must account for the law of conservation of mass. The amounts of reactants and products need to be adjusted so that the numbers and types of atoms are the same on both sides of the equation. This process is called balancing an equation and is done by inserting coefficients. Word and Formula Equations Give an example of how to balance an equation Additional Symbols Used in Chemical Equations Reversible reaction- a chemical reaction in which the products re-form the original reactants You should be able to interpret these symbols when they are used and to supply them when given the necessary information. Additional Symbols Used in Chemical Equations Additional Symbols Used in Chemical Equations Sample Problems Write word and formula equations for the chemical reaction that occurs when solid sodium oxide is added to water at room temperature and forms sodium hydroxide (dissolved in water). Include symbols for physical states in the formula equation. Then provide a balanced chemical equation. Sample Problems Translate the following chemical equation into a sentence: BaCl2(aq) + Na2CrO4(aq) BaCrO4(s) + NaCl(aq) Significance of a Chemical Equation Chemical equations are very useful in doing quantitative chemistry. Quantitative information revealed by a chemical equation: Significance of a Chemical Equation The coefficients indicate relative, not absolute, amounts of reactants and products A chemical equation usually shows the smallest numbers of atoms, molecules, or ions that will satisfy the law of conservation of mass….to get larger amounts, multiply each coefficient by the same number: Significance of a Chemical Equation CH4(g) + 2O2(g) CO2(g) + 2H2O(g) 20 molecules of methane would react with 40 molecules of oxygen to yield 20 molecules of carbon dioxide and 40 molecules of water Significance of a Chemical Equation The relative masses of the reactants and products can be determined from the reaction’s coefficients The reverse reaction for a chemical equation has the same relative amounts of substances as the forward reaction Significance of a Chemical Equation There is also important information about a chemical reaction that is not provided by a chemical equation. It does not give an indication of whether a reaction will actually occur. Experimentation forms the basis for confirming that a particular reaction will take place. Balancing Chemical Equations Identify the names of the reactants and the products, and write a word equation Write a formula equation by substituting correct formulas for the names of the reactants and the products Balance the formula equation according to the law of conservation of mass Count atoms to be sure that the equation is balanced Make sure the coefficients represent the smallest possible whole-number ratio of reactants and products Sample Problem Decomposition of Water Word Equation Formula Equation Sample Problem Write a balanced chemical equation for the reaction of zinc with aqueous hydrochloric acid. The reaction produces zinc chloride and hydrogen gas. Section 2: Types of Chemical Reactions Because thousands of chemical reactions occur in living systems, industrial processes, and chemical laboratories, it would be difficult to memorize all of the possible reactions. There are five basic types of chemical reactions. Section 2: Types of Chemical Reactions Understanding characteristics of these reactions can make it easier to predict the products of specific reactions. These reactions include: synthesis, decomposition, single-replacement, double-replacement, and combustion reactions. Synthesis Reactions synthesis reaction- also known as a composition reaction; two or more substances combine to form a new compound A + X AX A and X can be elements or compounds. AX is a compound. Synthesis Reactions Reactions of Elements with Oxygen and Sulfur Almost all metals react with oxygen to form oxides, forming oxides with the formula MO, where M represents the metal. 2Mg(s) + O2(g) 2MgO(s) Synthesis Reactions Group 1 metals form oxides with the formula M2O. (ex: Li2O) Group 1 and Group 2 elements have a similar reaction with sulfur to form sulfides with the formula M2S and MS. 16Rb(s) + S8(s) 8Rb2S(s) 8Ba(s) + S8(s) 8BaS(s) Synthesis Reactions Some metals, like iron, can produce two different oxides: 2Fe(s) + O2(g) 2FeO(s) 4Fe(s) + 3O2(g) 2Fe2O3(s) Synthesis Reactions Nonmetals also form oxides. S8(s) + 8O2(g) 8SO2(g) C(s) + O2(g) 2CO2(g) Synthesis Reactions Most metals react with halogens to form either ionic or covalent compounds. Group 1 metals react with halogens to form ionic compounds with the formula MX, where M is the metal and X is the halogen. 2Na(s) + Cl2(g) 2NaCl(s) 2K(s) + I2(g) 2KI(s) Synthesis Reactions Group 2 metals react with halogens to form ionic compounds with the formula MX2. Mg(s) + F2(g) MgF2(s) Sr(s) + Br2(l) SrBr2(s) Synthesis Reactions Fluorine is so reactive that it combines with almost all metals. 2Na(s) + F2(g) 2NaF(s) Synthesis Reactions Active metals are highly reactive. Oxides of active metals react with water to form metal hydroxides. CaO(s) + H2O(l) Ca(OH)2(s) Synthesis Reactions Many oxides of nonmetals in the upper right portion of the periodic table react with water to form oxyacids. SO2(g) + H2O(l) H2SO3(aq) Synthesis Reactions Certain metal oxides and nonmetal oxides react with each other for form salts. CaO(s) + SO2(g) CaSO3(s) Decomposition Reactions decomposition reaction- a single compound undergoes a reaction that produces two or more simpler substances AX A + X AX is a compound. A and X can be elements or compounds. Decomposition Reactions Decomposition reactions are the opposite of synthesis reactions. Most decomposition reactions take place only when energy in the form of heat or electricity is added. Decomposition Reactions The simplest kind of decomposition reaction is the decomposition of a binary compound into its elements. electricity 2H2O(l) 2H2(g) + O2(g) Decomposition Reactions electrolysis- decomposition of a substance by an electric current Oxides of the less-reactive metals in the lower center of the periodic table decompose into their elements when heated. Joseph Priestly discovered oxygen through such a reaction: ∆ 2HgO(s) 2Hg(l) + O2(g) Remember that the ∆ symbol indicates that heat has been applied to the reaction. Decomposition Reactions When a metal carbonate is heated, it breaks down to produce a metal oxide and carbon dioxide gas. ∆ CaCO3(s) CaO(s) + CO2(g) Decomposition Reactions All metal hydroxides, except those with Group 1 metals, decompose when heated to yield metal oxides and water. ∆ Ca(OH)2(s) CaO(s) + H2O(g) Decomposition Reactions When a metal chlorate is heated, it decomposes to produce a metal chloride and oxygen. ∆ 2KClO3(s) 2KCl(s) + 3O2(g) Decomposition Reactions Certain acids decompose into nonmetal oxides and water. H2CO3(aq) H2O(l) + CO2(g) ∆ H2SO4(aq) H2O(l) + SO3(g) Single-Displacement Reactions single-displacement reaction- also known as a replacement reaction; one element replaces a similar element in a compound A + BX AX + B or Y + BX BY + X A, B and X are elements. AX, BX, and BY are compounds. Single-Displacement Reactions The amount of energy involved in this type of reaction is usually smaller than the amount involved in synthesis or decomposition reactions. Single-Displacement Reactions The more active metal replaces the less active metal. 2Al(s) + 3Pb(NO3)2(aq) 3Pb(s) + 2Al(NO3)3(aq) Single-Displacement Reactions The most-active metals, like in Group 1, react vigorously with water to produce metal hydroxides and hydrogen. 2Na(s) + 2H2O(l) 2NaOH(aq) + H2(g) Single-Displacement Reactions Less-active metals, like iron, react with steam to form a metal oxide and hydrogen gas. 3Fe(s) + 4H2O(g) Fe3O4(s) + 4H2(g) Single-Displacement Reactions The more-active metals react with certain acidic solutions to replace the hydrogen in the acid. The products are a metal compound (a salt) and hydrogen gas. Mg(s) + 2HCl(aq) H2(g) + MgCl2(aq) Single-Displacement Reactions Here, one halogen replaces another halogen in a compound. Fluorine is the most-active halogen and can replace any of the other halogens in their compounds. Each halogen is less active than the one above it. So in Group 17, each element can replace any element below it, but not above it. Single-Displacement Reactions Cl2(g) + 2KBr(aq) 2KCl(aq) + Br2(l) F2(g) + 2NaCl(aq) 2NaF(aq) + Cl2(l) Br2(l) + KCl(aq) no reaction Double-Displacement Reactions double-displacement reactions- the ions of two compounds exchange places in aqueous solution to form two new compounds AX + BY AY + BX A, X, B and Y in the reactants represent ions. AY and BX represent ionic or molecular compounds. Double-Displacement Reactions One of the compounds formed is usually a precipitate, an insoluble gas that bubbles out of the solution, or a molecular compound, usually water. The other compound is often soluble and remains dissolved in solution. Double-Displacement Reactions The formation of a precipitate forms when the cations of one reactant combine with the anions of another reactant to form an insoluble or slightly soluble compound. The precipitate forms as a result of the very strong attractive forces between the cations and anions. 2KI(aq) + Pb(NO3)2(aq) PbI2(s) + 2KNO3(aq) Double-Displacement Reactions In some double-displacement reactions, one of the products is an insoluble gas that bubbles out of the mixture. FeS(s) + 2HCl(aq) H2S(g) + FeCl2(aq) Double-Displacement Reactions Sometimes a very stable molecular compound, such as water, is a product of a double-replacement reaction. HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) Combustion Reactions combustion reaction- a substance combines with oxygen, releasing a large amount of energy in the form of light and heat 2H2(g) + O2(aq) 2H2O(g) C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) How to Determine Reaction Types Section 3: Activity Series of the Elements activity- the ability of an element to react activity series- a list of elements organized according to the ease with which the elements undergo certain chemical reactions Section 3: Activity Series of the Elements The more readily an element reacts with other substances, the greater its activity is. For metals, greater activity means a greater ease of loss of electrons, to form cations. For nonmetals, greater activity means a greater ease of gain of electrons, to form anions. Section 3: Activity Series of the Elements The order in which the elements are listed is usually determined by singledisplacement reactions. The most-active element, placed at the top of the series, can replace each of the elements below it from a compound in a single-displacement reaction. An element farther down can replace any element below it but not above it. Section 3: Activity Series of the Elements Activity series help predict whether certain chemical reactions will occur. 2Al(s) + 3ZnCl2(aq) 3Zn(s) + 2AlCl3(aq) Co(s) + 2NaCl(aq) no reaction Sample Problems Zn(s) Sn(s) Cd(s) + + + Cu(s) + H2O(l) O2(g) Pb(NO3)2(aq) HCl(aq) ___________________ ___________________ ___________________ ___________________