* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CD8 T cell
Psychoneuroimmunology wikipedia , lookup
Immune system wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Molecular mimicry wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Innate immune system wikipedia , lookup
Adaptive immune system wikipedia , lookup
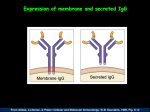
Adaptive Immunology Overview W. Robert Fleischmann, Ph.D. Department of Urologic Surgery University of Minnesota Medical School [email protected] (612) 626-5034 Objectives • Provide an overview of immunological principles • Provide a framework for future lectures • Introduce immunological terminology At age 3 months, Andrew Anderson begins to suffer from a series of bacterial infections that never seem to quite resolve with antibiotic treatment. Consequently, he has had nearly continual diarrheal and respiratory infections. As a result, he has not gained much weight since birth. At age 4 months, his parents bring him to the clinic with a white growth on the mucosal surface of his mouth. What do you think is wrong with Andy? What tests might you wish to request? Andy Anderson Total WBC count: Differential count: Neutrophils Lymphocytes Macrophages Eosinophils Basophils Immunoglobubin Levels: IgM 10 mg/100 ml IgG 100 mg/100 ml IgA 3 mg/100 ml 4,800/µl 79% 10% 10% 1% 0% (4,100-10,900/µl) (35-80%) (20-50%) (2-12%) (0-7%) (0-2%) (25-60 mg/100 ml) (275-600 mg/100 ml) (10-45 mg/100 ml) Adaptive Immunity Differs From Innate Immunity In Important Ways • Innate Immunity – Pre-existing defenses that are non-specific – Pre-existing defenses that do not change with repeated exposure • Adaptive Immunity – Reactive defenses that are specific – Reactive defenses that have memory and can give a greater level of 2° response Cells of the Adaptive Immune System • B and T Lymphocytes • Monocytes/Macrophages/Dendritic Cells • Basophils and Mast Cells • Eosinophils Lymphoid Organs • B cells and T cells are produced in the bone marrow. • B cells are released from the bone marrow as mature cells, while T cells must pass through the thymus to become mature cells. • Mature B cells and T cells can be in the blood or resident in the lymph nodes and spleen. – The spleen filters the blood – The lymph nodes filter the lymph • Mature B cells and T cells can travel from one lymph node to another and to and from the spleen (trafficking or homing). There Are Two Arms To Adaptive Immunity • Cell-Mediated Immunity Cytotoxic T cells • Humoral Immunity Antibodies Adaptive Immunity - Theory • Specific response for each antigen – Can recognize 107 to 109 different antigenic sequences – Only a few T and B lymphocytes recognize any given antigenic sequence • Must be induced – Requires 7-10 days for activation because rare B and T lymphocytes with identical antigen recognition sequences must find each other • Generates memory – Takes 1-5 days additional days for development of memory Adaptive Immunity - Overview • Antigen is phagocytosed and processed by professional antigen-presenting cells, such as macrophages, dendritic cells, and B cells. • An epitope of the antigen is bound to an MHC class II molecule and presented to the helper T cell. • The helper T cell produces cytokines and stimulates the activation of cytotoxic T cells and B cells. Functions of the Thymus • Immature T cells migrate from the bone marrow to the thymus. • The T cells mature to become either CD4+ helper T cells or CD8+ cytotoxic T cells. • During the maturation process selection occurs. – Positive selection: T cells must recognize MHC class I or MHC class II molecules in order to be stimulated to mature (self-restricted). – Negative selection: T cells that recognize self-antigens bound to MHC class I or MHC class II on thymus epithelial cells are driven to apoptose (tolerant to self-antigens. • Mature T cells that are self-MHC restricted and tolerant to self-antigens leave the thymus to settle in lymph nodes or the spleen. Functions of the Cells Involved in Adaptive Immunity Antigen Presentation • The first step in responding to an antigen is to be able to “see” it. • Macrophages and dendritic cells (also B cells) are professional antigen-presenting cells. – Macrophages are found in the tissues and lymph nodes. • Monocytes are undifferentiated macrophages that are found in the blood. – Dendritic cells are found in the tissues and lymph nodes. • Macrophages and dendritic cells produce important cytokines and lymphokines that activate T and B cells. Antigen Presentation • Antigens are presented as peptides (epitopes) bound to antigen-presenting molecules. • In the mouse, the antigen-presenting molecules are called major histocompatibility antigens or MHC. • In man, the antigen-presenting molecules are called human leukocyte antigens or HLA. What Is an Epitope? • Epitopes are regions on an antigen that can be recognized by an antibody or by T cell receptor. • Epitopes are also called antigenic determinants. • In the picture, epitopes on a whole poliovirus virion and on the isolated poliovirus VP1 protein are shown in white. Two Types of HLA Molecules Are Used for Antigen Presentation • HLA Class I Antigen Presentation – Antigens that are synthesized within a cell – Self-antigens or antigens from cell infection – Recognized by CD8+ cytotoxic T cells • HLA Class II Antigen Presentation – Antigens that are products of phagocytosis are expressed on HLA Class II antigens – Recognized by CD4+ helper T cells Antigen Presentation What Cells Express HLA? • HLA Class I – All cells except RBCs – Lack of expression on RBCs may play a role in persistence of malarial parasite (Plasmodium) • HLA Class II – – – – Monocytes/macrophages Dendritic cells B cells Epithelial cells of thymus Class I versus Class II HLA • Class I (one unique chain plus common chain [2microglobulin]) – Expresses epitopes of antigens (8-10 aa) that have been digested in the endoplasmic reticulum (endogenous antigen); 8-10 aa bound to 1 and 2 domains – Recognized by cytotoxic T cells – Important for killing virus-infected cells and for tumor surveillance – Mediates transplant rejection • Class II (two unique chains: chain plus chain) – Expresses epitopes of antigens (12-28 aa) that have been digested in phagolysosomes (exogenous antigen); 12-28 aa bound to 1 and 1 domains – Recognized by helper T cells to trigger adaptive immunity Characteristics of T Cells • All T cells express CD3. • All T cells express a T cell receptor that recognizes their cognate antigen epitope. • T cell receptors are created through DNA rearrangement; 1010 to 1012 paratopes. • There are two main subtypes of T cells. – CD4+ Helper T cell – CD8+ Cytotoxic T cell T Lymphocytes • T lymphocytes (T cells) – One subset expresses CD4 on their surface • CD4 = for something = help • CD4+ helper T cells play the central role in adaptive immunity as activators of both cellmediated and humoral immunity – One subset expresses CD8 • CD8 = ate • CD8+ cytotoxic T cells are effectors of cellmediated immunity that are HLA-restricted (see antigen in context of HLA) Activation of Helper T Cells • Signal 1 – Requires T cell receptor recognition of HLA bound antigen – Requires CD4+ binding to Class II or CD8+ binding to Class I • Signal 2 – B7-1 or B7-2 on the antigen presenting cells binds to T cell surface protein CD28 • Other co-stimulation molecules – CD2 binding to leukocyte functional antigen-3 (LFA-3) – Intercellular adhesion molecule (ICAM) binding to LFA-1 • Cytokine signals Cytokine Signals That Activate Helper T Cells • IL-2 and IL-15 are general activators of T cells. • IL-12 and IFN- drive T helper cells to Th1 helper cell subtype. • IL-4 drives T helper cells to Th2 helper cell subtype. • IL-10 down-regulates Th1 and TGF- down-regulate Th1 and Th2 subtypes. T Cell: APC Contacts Helper T Lymphocytes • CD4+ Helper T cells mature in the thymus. • CD4+ Helper T cells are activated by macrophage-produced or dendritic cellproduced cytokines. • Differential cytokine production by macrophages induces different types of helper T cells. – Th1 cells are induced by exposure of naïve CD4+ T cells to IL-1 + IL-12. – Th2 cells are induced by exposure of naïve CD4+ T cells to IL-1. Th1 Helper T Lymphocytes • CD4+ Th1 Helper T cells – Activated by IL-1 + IL-12 – Produce IL-2, IFN- – Activate CD8+ cytoxic T cells to divide and differentiate – Some CD4+ Th1 helper T cells become memory cells Th2 Helper T Lymphocytes • CD4+ Th2 Helper T cells – Activated by IL-1 – Produce IL-4, IL-5, IL-6, IL-9, IL-10, IL-13 – Activate B cells to divide and differentiate to become plasma cells that produce antibodies – Some CD4+ Th2 helper T cells become memory cells Regulatory T Lymphocytes • CD4+ Regulatory T cells (Tregs) – Produce IL-10 and TGF- – Down-regulate Th1 and Th2 cells functions, respectively – Some CD4+ Treg cells become memory cells Cytotoxic T Lymphocytes • CD8+ T cells mature in the thymus. • CD8+ T cells recognize target cells by antigen bound on MHC class I molecules. • CD8+ T cells kill target cells by several different mechanisms. • Some CD8+ T cells become memory cells. Killing by Cytotoxic T Lymphocytes • Kill by FAS - FAS ligand interaction – T cells expressing FAS ligand bind to FAS, a protein on the target cell, inducing caspase activation and apoptosis • Kill by secreting toxic agents – TNF, a cytokine, binds to TNF receptor on the target cell, inducing caspase activation and apoptosis – Perforin, a pore-forming protein, related to complement C9 is inserted into the target cell membrane causing lysis – Granzymes, serine esterases that activate apoptosis in a manner similar to that of caspases, are passed to target cells Complement Pores Versus Perforin Pores B Lymphocytes • B lymphocytes (B cells) – Express CD19, CD20, and CD22 on their surface – Differentiate to plasma cells that produce antibodies – Some B cells become memory B cells B Lymphocytes • Rearrange their DNA as they mature in bone marrow – Each B cell has a unique VDJ rearrangement (idiotype) – Antibody repertoire permits recognition of “all” antigens • When activated, divide and differentiate to become plasma cells – Undergo class switching from IgM or IgD by recombination • Change from IgM or IgD isotype production to IgG, IgE, or IgA production – Express increased affinity for antigen (affinity maturation) • Undergo somatic cell mutations as they differentiate • Undergo sloppy recombination during isotype switching Antibody Structure Antibody Rearrangement Terms Related to Antibodies • Epitope: part of antigen that binds to antibody • Paratope: part of antibody that binds antigen • Idiotype: unique antigen-binding site on each antibody; antibodies with same antigen binding sites have the same idiotype • Isotypes: antibodies with different types of Fc regions (i.e., IgM, IgD, IgG, IgE, IgA) • Allotypes: antibodies from different individuals that have slightly different Fc regions (i.e., different IgM) B Cell Proliferation • Each B cell expresses specific antibody on its surface that recognizes a specific antigen. • B cells are triggered to proliferate and differentiate to plasma cells by exposure to that specific antigen. • Triggering this proliferation also requires help by exposure to CD4+ Th2 cytokines. • Plasma cells then secrete this specific antibody. • For memory B cells, exposure to antibody alone is sufficient. Types of Antibodies IgD: Membrane-associated Ig that is found on mature but unactivated B cells IgM: Pentameric Ig that is found on mature but unactivated B cells; first secreted Ig made; found in blood IgG: Monomeric Ig that is most abundant Ig in blood and most important Ig for memory response IgE: Monomeric Ig that is associated with mast cells and basophils; mediator of allergic reactions IgA: Dimeric Ig that is associated with J chain and secretory component; most important secretory Ig Stylized Structures Of Different Antibody Classes Summary of Acquired Immunity M IL-1 IL-12 IL-1 CD4 Th1 CD4 Th2 IL-2 IL-3 IFN- IL-4 IL-5 IL-6 IL-9 IL10 IL-13 CD8 T cell Cytolysis: FAS/FAS ligand TNF Granzyme B Perforin B cell Antibody Andy Anderson Culture of the white growth showed the presence of Candida albicans. Lymphocyte stimulation test: Phytohemaglutinin1 4.28 134.3 Concanavalin A1 1.33 48.8 Pokeweed mitogen2 1.56 46.5 NK cell test: normal activity of NK cells What do these results indicate? What treatment can be performed? 1 T cell mitogen 2 B cell mitogen Andy Anderson Andy has severe combined immunodeficiency disorder. Causes of SCID: No T cells: IL-7 -chain deficiency CD3 -chain deficiency No T cells or B cells: RAG1 or RAG2 deficiency (no TCR/Ab rearrangement) No T cells or NK cells: X-linked IL-3R -chain deficiency JAK-3 deficiency CD45 deficiency No T cells, B cells, or NK cells: Adenosine deaminase (ADA) deficiency Purine nucleoside phosphorylase deficiency Andy Anderson Andy is found to have a RAG1 deficiency that is causing his severe combined immunodeficiency disease. Andy is given a bone marrow transplant from his father. He recovers from the bone marrow transplant to have a reconstituted immune system. Note: Since 2010, testing for SCID is part of the neonate genetic testing program in every state in the U.S. Additional Topics • Most important disorders of the adaptive immune system • Hypersensitivities • Tolerance B Cell Immunodeficiencies • Disorders of B cells – Bruton’s agammaglobulinemia (x-linked agammaglobulinemia) – Transient hypogammaglobulinemia of infancy – Common variable immunodeficiency (actually defect in T cell signaling of B cell) – IgA and IgG subclass deficiencies T Cell Immunodeficiencies • Disorders of T cells – Severe combined immunodeficiency syndrome (SCID) (common chain lack of IL-7 signaling causes failure to mature) – Purine nucleoside phosphorylase deficiency (causes severe combined immunodeficiency) – Adenosine deaminase deficiency (causes severe combined immunodeficiency) – MHC class II deficiency – DiGeorge’s syndrome (congenital thymic aplasia) – Wiscott-Aldrich Syndrome (x-linked, few platelets, cytotoxic T cell malfunction) – Ataxia-telangiectasia (wobbly gait, T cell deficiency) Deficiencies of Innate Immunity • Congenital neutropenia – Lack of GM-CSF – Frequent bacterial infections • Glucose-6-phosphate dehydrogenase deficiency (G6PD) – Unable to produce NADPH by pentose phosphate pathway, buildup of reduced glutathione – RBC denaturation and hemolysis • Chronic granulomatous disease – Inability to produce hydrogen peroxide and hypochlorous acid – Inability to kill phagocytosed bacteria • Leukocyte adhesion deficiency (LAD) – Lack of integrin subunit, the common chain – Inability to recruit innate immune cells to site of inflammation – Increased susceptibility to bacterial, fungal, and viral infections. • Complement defects – Increased susceptibility to bacterial infections – Reduced ability to remove immunocomplexes • Chediak-Higashi Syndrome – Defect in gene LYST (CHS1), a lysosomal trafficking gene that affects lysosomes and melanosomes (microtubule disorder) – Increased susceptibility to bacterial infections. Hypersensitivities • Type I Hypersensitivity – Allergy – Mediated by IgE bound to mast cells and to basophils causes degranulation (early phase) – Release of histamine – Synthesis of prostaglandins and leukotrienes – Recruitment of Th1 cells and basophils (late phase) Hypersensitivities • Type II Hypersensitivity – Antibodies against cell surface and/or extracellular matrix components – Causes complement activation – Examples • hemolytic disease of the newborn (maternal antibodies to fetal blood group antigens cross the placenta to destroy the fetal RBCs) • Myasthenia gravis (antibodies to acetylcholine receptors cause problems with nerve conduction) • Goodpasture’s syndrome (antibodies to basement membranes causes nephritis) Hypersensitivities • Type III Hypersensitivity – Immune complex disease – Deposition of antigen:antibody complexes • Kidney, joints, lungs, arteries, skin • Damage occurs by activation of complement – Examples • Post-streptococcal glomerulonephritis • Autoimmunity such as Systemic Lupus Erythrematosus Hypersensitivities • Type IV Hypersensitivity – Cell-mediated hypersensitivity caused by activated CD4 cells – Examples • Contact hypersensitivity such as reaction to nickel – Dendritic Langerhans cells react recruiting CD4 cells that ultimately mediate this hypersensitivity • Tuberculin reaction – Macrophages react recruiting CD4 cells (2/3) and CD8 cells (1/3) that ultimately mediate this hypersensitivity • Granulomatous hypersensitivity – Macrophages wall off mycobacterium, undergo changes to become epitheloid (that may form giant cells), and recruit CD4 cells. – Crohn’s disease = granulomas containing macrophages and CD4 cells in the ileum and colon Tolerance • Central Tolerance – Self-reactive T cells recognize antigens presented by thymus epithelial cells and are deleted in the thymus by negative selection – Self-reactive B cells are deleted in the bone marrow • Peripheral Tolerance – Not all self-antigens can be presented in the thymus – Thus, tolerance must be established in mature T and B cells Possible Mechanisms of Peripheral Tolerance • Clonal exhaustion – High levels of self-antigen expression trigger a rapid proliferative response in responding T cells that cannot be sustained in the absence of cytokines and results in apoptosis of the responding T cells (no cytokinemediated activation of T cells) • Clonal anergy – Non-professional antigen presenting cells or resting (non-activated) antigen presenting cells provide only signal one but not signal two (B7 molecules), so no proliferation (no cytokine-mediated activation by APCs) • Regulatory T cells – CD4+ T cells that also are CD25+ inhibit Th1 and Th2 cells (IL-10- and TGF--mediated inhibition)