* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download (P>0.05)。
Survey
Document related concepts
DNA vaccination wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Molecular mimicry wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Neonatal infection wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Henipavirus wikipedia , lookup
Innate immune system wikipedia , lookup
Pathophysiology of multiple sclerosis wikipedia , lookup
Hepatitis C wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Multiple sclerosis signs and symptoms wikipedia , lookup
Infection control wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Transcript
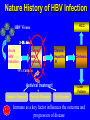
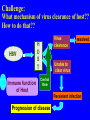
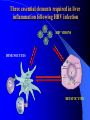
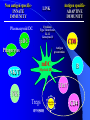
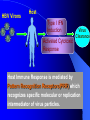
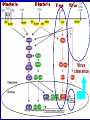
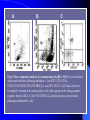
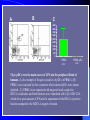
Pattern Recognition Receptors and HBV Infection Role of Toll-like Receptors Dept of Infectious Diseases Shanghai Ruijin Hospital Jiaotong University School of Medicine Qing Xie HBV infection is still a major global health care problem High prevelance Widely spread • 350 million chronic carriers worldwide • 9th leading causes of death • 75% of HBV carrier are Asian HBsAg rate (%) 8: high 2-7: medium <2: low Nature History of HBV Infection HCC HBV Virons 5年 3-5% Adult Chronic HBV Infection Acute HBV Infection Chronic 12-20% Cirrhosis Hepatitis B 5年 95% Children 5年 Antiviral treatment Complete Response Partially Response 6-15% 20-23 Liver Failure Non Response Immune as a key factor influences the outcome and progression of disease Challenge: What mechanism of virus clearance of host?? How to do that?? HBV Immune function of Host Virus clearance H O S T resolved Unable to clear virus Central Role Persistent infection Progression of disease Three essential elements required in liver inflammation following HBV infection HBV VIRONS IMMUNOCYTES HEPATOCYTES Non antigen specificINNATE IMMUNITY Plasmacytoid DC pDC Antigen specificADAPTIVE IMMUNITY LINK Cytokines Type I interferon IL-12 Granzyme B CD8 Antigen presentation Phagocytes mDC NK/T B Myeloid DC Th17 NK Tregs 调节性T细胞 CD4+CD25+ Treg cell CD4 HBV Virons Host Type I IFN Induction Activated Cytotoxic Response Virus Clearance Host Immune Response is mediated by Pattern Recognition Receptors(PRR) which recognizes specific molecular or replication intermediator of virus particles. Pattern Recognition Receptors(PRR) Recent Studies found: The close relationship between host innate immunity and the recognition of pathogen and clearance. TLR as an important PRR,plays a central role in defence of virus invasion. Different TLR can recognize various pathogen. G+bacteria G-bacteria Virus Virus Virus clearance What is a role of TLR in HBV infection?? HBV G+bacteria G-bacteria Disturbance of TLR expression induces the persistent infection. Virus clearance Abnormal of signal passway initiated by TLR induces the inability to clear HBV or overclearance, that leads to immunotolerance or immune injury. Requirement of DC involment in TLR-initiated antivirus response Toll-like receptor (TLR)-mediated recognition of specific structures of invading pathogens initiates innate as well as adaptive immune responses via antigen-presenting cells (APCs). DC represents the most potent antigen-presenting cells and thus plays an important role in the induction of innate and specific immune response. Distribution of TLR on DCs mDC: (myeloid dendritic cell,mDC) ( CD3、CD14、CD16、CD19、CD20、CD56) -BDCA1(CD1c)+ CD11c+ TLR1 TLR2 TLR3 TLR4 TLR5 TLR6 TLR8 pDC:(plasmacytoid dendritic cell, pDC) ( CD3、CD14、CD16、CD19、CD20、 CD56)-BDCA2+ BDCA4+ CD123+ TLR7 TLR9 TLR3、TLR7、TLR9 参与抗病毒免疫 Scientific Questions? Function of Myeloid and Plasmacytoid Dendritic Cells of Patients with CHB?? Expression of TLR-3, TLR-9 in patients following HBV chronic infection?? Study Populations CHB: 28 cases Healthy:22 cases Including criteria: (1) Serum HBsAg positive > 6 months. (2) Abnormal liver function for 2 times with 2 weeks apart, ALT>1.5 xU/L at screeing. (3) Serum HBeAg(+),HBeAb(-). (4) Serum HBV-DNA>1×105 /L. (5) excluded liver cirrhosis by liver ultrasound (6) excluded HIV、HCV、HDV、HEV coinfection. (7) Not allowed to use antiviral treatment or immunmodulator within 6 months. Selection and preparation of pDC、mDC pDC BDCA4 DC isolation kir mDC BDCA1 DC siolation kit p<0.05 p>0.05 positive cell % 125 600 500 400 300 200 100 0 100 MFI 75 50 25 0 patients n=28 controls n=18 patients n=28 controls n=18 Fig.3. TLR9 expression of isolated peripheral precursor pDCs of chronic HBV patients (n=28) and healthy controls (n=18). (A) No difference in the percentage of pDCs expressing TLR9 was found between the patients and the controls. (B) The MFI of TLR9 from patients were significantly reduced compared to controls. Data are expressed as mean±SD A B C Fig.4. Flow cytometric analysis for enumeration of pDCs. PBMCs were isolated and stained with the following antibodies:Lin-FITC( CD3,CD14, CD16,CD19,CD20,CD56), PE-BDCA-2, and APC-CD123. (A) Dead cells were excluded by forward side scatter analysis. (B) After gating on the lineage markernegative fraction (R2), (C) the CD123/BDCA-2-double positive represents the plasmacytoid dendritic cells. A B p<0.05 600 r =-0.646 p=0.02 500 0.50 ALT??(IU/L) pDC frequency% 0.75 0.25 400 300 200 100 0 0.00 CHB n=21 NS n=26 0.0 0.1 0.2 0.3 0.4 0.5 pDC/PBMC% Fig.5. (A)In patients with CHB the relative numbers of pDCs were significantly reduced compared healthy controls(P<.05). (B) Correlation of the relative frequency of pDCs with the ALT levels in chronic HBV infection. The relative counts of pDCs were inversely correlated with ALT values ( P<.05; r﹦-0.645). B C IFNa(pg/L) A 4500 4000 3500 3000 2500 2000 1500 1000 500 0 PBMC n=22 PBMC-pDC n=22 Fig.6. pDCs were the main source of IFN-αin the peripheral blood of humans. (A) An example of frequency analysis of pDCs in PBMCs. (B) PBMCs were analyzed by flow cytometer which showed pDCs were almost depleted. (C) PBMCs were separated with magnetic beads coupled to BDCA-4 antibodies and both fractions were stimulated with CpG-ODN 2216 , which show great amount of IFN-αin the supernatant of the BDCA-4-positive fraction compared to the BDCA-4-negative fraction. C B A p<0.05 p>0.05 p>0.05 3000 2000 10000 0 2000 IL-6 pg/ml 20000 TNF pg/ml IFN-a pg/ml 30000 1000 1000 0 0 patients n=22 controls n=20 patients n=22 controls n=20 patients n=22 controls n=20 Fig.7. Cytokine production by isolated peripheral precursor pDCs of chronic HBV patients (n=22) and healthy controls (n=20) after stimulation with CpGODN 2216. After 24 hours of stimulation, cytokine production was determined in the culture supernatants by specific ELISAs. (A) pDCs of patients were significantly impaired in their ability to produce IFN-αcompared to healthy controls. No difference was detected in the production of (B) TNF-αand (C) IL-6 between patients and healthy controls. The decrease of TLR9 expression ability by DC is related to HBV infection in DC? Hepatology 2006;43(3):539-547 HBsAg HBcAg CONCLUSIONS 1. The TLR9 expression of circulating pDCs is reduced in patients with CHB. 2. pDCs are functionally impaired with the lower ability to produce IFN-α in patients, that may partly contribute to hepatitis B evading an adequate immune response, resulting in HBV persistent infection in host. 3. HBsAg and HBcAg were detectable in pDCs of patients suggest that functional impairment of pDCs may correlate with HBV infection of pDCs. 12h 0h 24h 48h 24h The expressions of TLR3(%) 0h 12h 48h 100 CH HV 80 60 0h 12h 24h 48h Time Fig8. The levels of TLR3 expression on mDC of peripherial blood between contrl and patients. It shows that TLR3 level in control is increased following 24 hrs stimulaton, however the increase of TLR3 expression was delayed to 48 hrs following stimulation. P<0.05。 0.5 0.45 0.4 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 CH HV * * 0h 12h 24h 48h Fig9. The changes of TLR3 mRNA by real-time PCR at various timepoints following stimulation.It shows TLR3 mRNA is increased significantly at 12 hrs, it recovers to the baseline at 24 hrs and 48 hrs. However the increase of TLR3 mRNA in CHB patients was observed at 48 hrs. *P<0.05。 1.2 1 0.8 CH HV 0.6 * 0.4 0.2 0 0h 12h 24h 48h Fig10. Expression of TBK1 mRNA on mDC by RT-PCR. There is no difference in TBK1 mRNA at baseline between control and patients (P>0.05)。TBK1 mRNA is increased significantly at 12 hrs when compared to baseline in control (P<0.05). There is no difference in TBK1 mRNA at 12hrs, 24hrs and 48hrs when conpared to baseline (P>0.05)。 0.18 0.16 0.14 0.12 0.1 0.08 0.06 0.04 0.02 0 CH HV * 0h 12h 24h 48h Fig11. Expression of IFN-βmRNA by RT-PCR in mDC. There is no difference in at baseline between control and patients (P>0.05)。 The level of IFN-βmRNA expression at 12 hrs is higher than at baseline in control (P<0.05). However there is no deifference between at various time points(P>0.05)。 The IFN-beta(pg/ml) 200 180 160 140 120 * 100 80 CH HV 60 40 20 0 0h 24h 12h 48h Time Fig12. The detection of IFN-β by ELISA in supernatant on mDC following polyI:C stimulation.The level of IFN-β at baseline is very similar between two groups.There is no difference at various timepoints following stimulation in patients.However,the level of IFN-βis much higher at 12 hrs when compared to baseline.Also the difference was observed at 12hrs, 24hrs and 48 hrs between control and patients. CONCLUSIONS The increase of expression level of TLR3 is slower and delayed in CH groups than HV groups. It contributes to the inability for the host to clear HBV. The level of TBK1 molecular is quite low even following dsRNA stimulation, suggesting that abnormal of signal transduction passway may exist in HBV . Our results suggest that dysfunction of TLR3 might play an important role in chronic HBV infection. It may provide new insights for understanding the mechanism of persistent HBV infection