* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Thesis_Panitz
Immune system wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Adaptive immune system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Ulm University
Department of Pediatrics and Adolescent Medicine
Director:
Prof. Dr. med. Klaus-Michael Debatin
Regulation of human granzyme Bproducing plasmacytoid dendritic cells
by viral stimuli
Dissertation
submitted in fulfillment of the requirements for the degree of
Doctor of Medicine (Dr. med.)
to the Faculty of Medicine of Ulm University
by
Verena Michaela Panitz
born in Ulm, Germany
2015
Acting Dean:
Prof. Dr. rer. nat. Thomas Wirth
First Examiner:
PD Dr. med. Dorit Fabricius
Second Examiner:
Prof. Dr. med. Marion Schneider
Date of Graduation:
November 18th, 2016
Parts of this dissertation have already been published in the
following journal articles:
The Journal of Immunology:
Fabricius, D, Nussbaum, B, Busch, D, Panitz, V, Mandel, B, Vollmer, A, Westhoff,
MA, Kaltenmeier, C, Lunov, O, Tron, K, Nienhaus, GU, Jahrsdorfer, B, and
Debatin, KM: Antiviral vaccines license T cell responses by suppressing granzyme
B levels in human plasmacytoid dendritic cells. J Immunol 191: 1144-1153 (2013)
doi:10.4049/jimmunol.1203479
Journal of Vaccines & Vaccination:
Jahrsdörfer, B, Panitz, V, and Fabricius, D: Human Immunodeficiency Virus
Arrests Plasmacytoid Dendritic Cells in a Granzyme Bhigh Tolerogenic State. J
Vaccines Vaccin 7: 337 (2016)
doi:10.4172/2157-7560.1000337
For my family
Table of contents
List of abbreviations ................................................................................................... VII
1
Introduction ............................................................................................................. 1
1.1 Plasmacytoid dendritic cells- mediators between innate and adaptive
immunity ............................................................................................................................. 1
1.2 Granzyme B- new roles and functions ........................................................................... 4
1.3 Plasmacytoid dendritic cell-derived granzyme B ......................................................... 5
1.4 Clinical importance of viruses used in this study ......................................................... 6
1.5 Hypothesis and aims ........................................................................................................ 9
2
Material and Methods............................................................................................ 10
2.1 Samples ............................................................................................................................ 10
2.2 Buffers ............................................................................................................................... 10
2.3 Viral stimuli ....................................................................................................................... 11
2.4 Isolation of specific cell subsets.................................................................................... 13
2.5 Cell culture ....................................................................................................................... 18
2.6 Enzyme-linked immunosorbent assay ......................................................................... 21
2.7 Flow cytometry................................................................................................................. 23
2.8 Data analysis and statistics ........................................................................................... 26
3
Results ................................................................................................................... 27
3.1 Herpesviruses modulate granzyme B production and secretion by
plasmacytoid dendritic cells in a concentration-dependent manner ....................... 27
3.2 Human immunodeficiency virus 1 increases granzyme B secretion by
plasmacytoid dendritic cells ........................................................................................... 31
3.3 Regulation of granzyme B production and secretion by plasmacytoid
dendritic cells is virus-specific ....................................................................................... 33
3.4 Interferon-α secretion by plasmacytoid dendritic cells is differentially
regulated by viruses ........................................................................................................ 34
V
3.5 Herpesviruses modulate the expression of distinct surface molecules on
plasmacytoid dendritic cells ........................................................................................... 37
3.6 Herpesviruses and human immunodeficiency virus 1 in the concentrations
or multiplicities of infection used do not relevantly alter viability of
plasmacytoid dendritic cells ........................................................................................... 41
3.7 The viral components of the different herpesvirus sources are responsible
for the effects on plasmacytoid dendritic cells ............................................................ 45
3.8 Herpesvirus- or human immunodeficiency virus 1-stimulated plasmacytoid
dendritic cells modulate CD4+ T cell proliferation in mixed lymphocyte
reactions ........................................................................................................................... 49
4
Discussion ............................................................................................................. 54
4.1 Virus-specific regulation of granzyme B in plasmacytoid dendritic cells ................ 54
4.2 Interaction of viruses with plasmacytoid dendritic cells ............................................ 56
4.3 Modulation of plasmacytoid dendritic cell interferon-α production and
phenotype by viruses ...................................................................................................... 60
4.4 The role of plasmacytoid dendritic cells in anti-viral immunity and viral
pathogenicity .................................................................................................................... 65
4.5 Granzyme B and tolerogenic plasmacytoid dendritic cells in anti-viral
immunity ........................................................................................................................... 74
4.6 Outlook.............................................................................................................................. 77
5
Summary ................................................................................................................ 78
6
References ............................................................................................................. 80
VI
List of abbreviations
7-AAD
7-amino-actinomycin D
Ab
Antibody
ACK
Ammonium chloride potassium
AIDS
Acquired immunodeficiency syndrom
AIM-V
Adoptive Immunotherapy Media
APC
Allophycocyanin
B7-H1
B7 homolog 1
BDCA
Blood dendritic cell antigen
BID
BH3 interacting domain death agonist
BSA
Bovine serum albumin
CCL
Chemokine (C-C motif) ligand
CCR
C-C chemokine receptor
CD
Cluster of differentiation
CD40L
Cluster of differentiation 40 ligand
CD45RA
Cluster of differentiation 45RA
CFSE
Carboxyfluorescein diacetate succinimidyl ester
CMV
Cytomegalovirus
cmvIL-10
Cytomegalovirus IL-10
CO2
Carbon dioxide
CpG
Cytosine-phosphate-guanine
CpG A
Class A cytosine-phosphate-guanine
oligodeoxynucleotide
CpG B
Class B cytosine-phosphate-guanine
oligodeoxynucleotide
CpG C
Class C cytosine-phosphate-guanine
oligodeoxynucleotide
CTL
Cytotoxic T lymphocyte
CXCL
Chemokine (C-X-C motif) ligand
CXCR
C-X-C chemokine receptor
d
Day
DC
Dendritic cell
DNA
Deoxyribonucleic acid
VII
dsDNA
Double-stranded deoxyribonucleic acid
EBV
Epstein-Barr virus
EDTA
Ethylenediaminetetraacetic acid
ELISA
Enzyme-linked immunosorbent assay
ER
Endoplasmic reticulum
FACS
Fluorescence activated cell sorting
FBS
Fetal bovine serum
FcR
Fc receptor
FCS
Fetal calf serum
FGFR1
Fibroblast growth factor receptor 1
FITC
Fluorescein isothiocyanate
Flt3-L
Fms-like tyrosine kinase 3 ligand
g
Gravitational acceleration
GluR
Glutamate receptor
GrB
Granzyme B
h
Hour
H2SO4
Sulphuric acid
HAART
Highly active anti-retroviral therapy
HCl
Hydrochloric acid
HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
HFF
Human foreskin fibroblast
hIL-10
Human IL-10
HIV
Human immunodeficiency virus
HIV-1
Human immunodeficiency virus 1
HLA
Human leukocyte antigen
HRP
Horseradish peroxidase
ICAM-1
Intercellular adhesion molecule 1
ICOS-L
Inducible T cell co-stimulatory-ligand
IDO
Indoleamine 2,3-dioxygenase
IE
Immediate-early
IFN
Interferon
Ig
Immunoglobulin
IL
Interleukin
IL3Rα
Interleukin-3 receptor α-chain (CD123)
VIII
IRF7
Interferon regulatory factor 7
ISG
Interferon-stimulated gene
KHCO3
Potassium bicarbonate
LFA-1
Leukocyte function-associated antigen 1
Lin 1
Lineage 1, cocktail containing antibodies against
CD3, CD14, CD16, CD19, CD20, CD56
mAb
Monoclonal antibody
MACS
Magnetic activated cell sorting
MAPK
Mitogen-activated protein kinase
min
Minute
MLR
Mixed lymphocyte reaction
MOI
Multiplicity of infection
mRNA
Messenger ribonucleic acid
MyD88
Myeloid differentiation primary-response gene 88
NaOH
Sodium hydroxide
NF-B
Nuclear factor-kappa B
NH4Cl
Ammonium chloride
NK cell
Natural killer cell
ODN
Oligodeoxynucleotide
ODN 2006
Class B cytosine-phosphate-guanine
oligodeoxynucleotide, sequence: 5’-tcg tcg ttt tgt cgt
ttt gtc gtt-3’ on phosphorothioate backbone
ODN 2336
Class A cytosine-phosphate-guanine,
oligodeoxynucleotide, sequence: 5’-g*g*g gac gac
gtc gtg g*g*g* g*g*g-3’ (*phosphorothioate bonds,
rest are phosphodiester bonds)
PAMP
Pathogen-associated molecular pattern
PBMC
Peripheral blood mononuclear cell
PBS
Phosphate buffered saline (without calcium and
magnesium)
pDC
Plasmacytoid dendritic cell
PE
Phycoerythrin
PE-Cy5
Phycoerythrin tandem with cyanin dye 5
IX
PerCP-Cy5.5
Peridinin chlorophyll protein tandem with cyanin
dye 5.5
PFA
Paraformaldehyde
PHA
Phytohemagglutinin,
lectin
used
for
mitogenic
stimulation of lymphocytes
PI
Propidium iodide
PKR
Protein kinase R
PRR
Pattern recognition receptor
RIG-I
Retinoic acid-inducible gene 1
RLH
RIG-I-like helicase
RPMI
Roswell Park Memorial Institute
RT
Room temperature
SIV
Simian immunodeficiency virus
ssRNA
Single-stranded ribonucleic acid
SV40
Simian virus 40
TBEV
Tick-borne encephalitis virus
TCR
T cell receptor
TLR
Toll-like receptor
TMB
Tetramethylbenzidine
TNF-α
Tumor necrosis factor-α
Tr1
Regulatory T1 cell
Treg
Regulatory T cell
UV
Ultraviolet light
UV-CMV
Ultraviolet light-inactivated cytomegalovirus in the
section discussion
VEGF
Vascular endothelial growth factor
vIL-10
Viral IL-10
Vpr
Viral protein R
VSV
Vesicular stomatitis Indiana virus
VZV
Varicella-zoster virus
w/o
Without
X
1 Introduction
1.1 Plasmacytoid dendritic cells- mediators between innate and
adaptive immunity
Dendritic cells (DCs) were first discovered in 1973 by Ralph M. Steinman and
Zanvil A. Cohn [162]. DCs are known as major players in innate and adaptive
immunity that have a decisive influence on the reactions of the immune system.
The diverse subsets exert different functions as professional antigen-presenting
cells that produce either pro-inflammatory or immunoregulatory cytokines and
interferons (IFNs) [158] and decide that way over immunity or tolerance. DCs can
be roughly subdivided in classical/ myeloid DCs and plasmacytoid DCs [178].
Plasmacytoid dendritic cells (pDCs), formerly known as professional type 1
interferon-producing cells [159], are a very rare blood cell subset representing only
0.2-0.8% of peripheral blood mononuclear cells (PBMCs) [109]. They were first
isolated in human tonsils in 1997 [64]. The corresponding mouse cell was
identified in 2001 [6, 14, 130]. Their name originates from their plasma cell-like
morphology in steady-state and their potential to develop into dendritic cells upon
maturation [64]. Human pDCs are characterized by the expression of the following
surface molecules: Cluster of differentiation (CD)4+, CD45RA+, human leukocyte
antigen (HLA)-DR+, CD123 (Interleukin-3 receptor α-chain (IL3Rα)high, CD11c-,
lineage (lin) 1-. Lin 1- means that pDCs do not express several surface markers
that characterize other immune cells: PDCs are negative for the T cell antigen
CD3, for CD14 (on monocytes), CD16, CD56 (on natural killer (NK) cells), CD19
and CD20 (on B cells). Human blood pDCs can be further distinguished by their
exclusive expression of blood dendritic cell antigen (BDCA)-2 and -4 (neuropilin-1)
[44].
PDCs are constantly produced from bone marrow [15], depending on the growth
factor fms-like tyrosine kinase 3 ligand (Flt3-L) [178]. Whether pDCs originate from
the lymphoid or myeloid pathway is to date not clear since pDCs can be derived
from both common lymphoid and myeloid progenitors [36, 157]. After production,
pDCs circulate in the blood and migrate via high endothelial venules into T cell rich
areas of secondary lymphoid organs such as lymph nodes [26], mucosaassociated lymphatic tissues and the spleen [109]. The life span of the murine
counterpart of human pDCs is about two weeks [137]. As human pDCs die rapidly
1
in cell culture, interleukin (IL)-3 is often supplemented, representing a crucial
survival factor for pDCs and therefore prolonging pDC survival ex vivo [64].
Physiologically, IL-3 is produced by sources such as activated T cells [65] or mast
cells [143].
The key features of pDCs are the expression of the intracellular pathogen
recognition receptors (PRRs) Toll-like receptor (TLR)7 and TLR9 [89, 92]. The
pathogen-associated molecular patterns (PAMPs) that are recognized by these
receptors are single-stranded ribonucleic acid (ssRNA) from viruses [39, 72, 115]
and synthetic small molecules such as imidazoquinoline compounds [74] or
loxoribine [71] for TLR7 and double-stranded deoxyribonucleic acid (dsDNA)
containing unmethylated 2’deoxyribo-cytosine-phosphate-guanine (CpG) DNA
motifs [9, 67, 73] for TLR9. The latter are common in bacterial and viral DNA and
are
included
in
synthetically
produced
immunostimulatory
CpG
oligodeoxynucleotides (ODN) that also act via TLR9 [96].
The signaling cascade downstream of TLR7 and TLR9 through the adaptor
molecule myeloid differentiation primary-response gene 88 (MyD88) [2] leads to
the rapid secretion of high amounts of type I interferons (IFNs), namely IFN-α, by
pDCs. PDCs are the main producers of type I interferons in the immune system as
they secrete up to 1000 times more type I IFN than any other cell upon appropriate
stimulation [159]. This is possible due to the constitutively high expression of
interferon-regulatory factor (IRF)7 [86] and a potent endoplasmic reticulum (ER)
machinery in pDCs. Moreover up to 60% of the transcriptional machinery of pDCs
is involved in producing type I IFNs [84]. IFNs induce the transcription of
interferon-stimulated genes (ISGs) in diverse cell types. The expression of these
anti-viral molecules makes the cells more resistant to viral infection as the cells
become more sensitive for detection and elimination of entering viruses or the antiviral molecules directly interact with viral replication or promote apoptosis of virally
infected cells [3].
Type I IFNs derived from pDCs can also boost innate and adaptive immune
responses as they induce the maturation of classical DCs [152] and activate
natural killer (NK) cells and cytotoxic T lymphocytes (CTLs) [3].
TLR7 and TLR9 can furthermore signal through nuclear factor-kappa B (NF-κB)
and mitogen-activated protein kinases (MAPKs) inducing the expression of costimulatory molecules such as CD80 and CD86 on the cell surface of pDCs and
2
the secretion of IL-6, Tumor-necrosis factor (TNF)-α and chemokines that recruit
immune cells to the sites of infection [66, 164]. For example, pDCs can attract NK
cells and activated T cells via secretion of chemokine (C-C motif) ligand (CCL)4
and chemokine (C-X-C motif) ligand (CXCL)10 [121]. In addition pDCs are able to
promote the differentiation of B cells to antibody-producing plasma cells [90].
Besides, maturated pDCs are able to present antigens on HLA-DR and HLA-ABC
molecules and can thereby initiate an adaptive immune response via antigen
presentation to CD4+ T cells [175] or via rapid cross-presentation of antigens to
CD8+ T cells in a proteasome-independent pathway [38]. Altogether, these
properties enable pDCs to activate and link both innate and adaptive immunity
especially in the course of viral infections.
PDCs are not only important in anti-viral immune responses, they also play a role
in several other diseases such as auto-immune diseases where they are found in
the peripheral organs. PDCs have been found to infiltrate the synovia of patients
with rheumatoid arthritis [24], skin lesions of patients with psoriasis [134] and of
systemic lupus erythematosus patients [16, 48], where pDCs play a pivotal role in
the pathogenesis as the major producers of type I IFNs [149], but also in the
central nervous system in a mouse model for multiple sclerosis where pDCs rather
have a regulatory function [7].
PDCs also infiltrate several tumors such as melanoma [173], breast cancer where
infiltration goes along with bad prognosis [169] and ovarian cancer [182] where
pDCs possibly play a role in immune evasion and tolerance induction to tumor
cells [176].
Lastly, pDCs play an important role in the maintenance of both peripheral as well
as central tolerance [68, 120]. For example, pDCs stimulated with different classes
of CpG ODNs induced regulatory T cells (Tregs) [128] via expression of
indoleamine 2,3-dioxygenase (IDO) [30].
In this study we assess the role of pDCs in the anti-viral immune response. The
real clinical relevance of human pDCs for viral infections has not been extensively
studied in the human system as pDCs display a rare immune cell subset. Study
results from mouse models cannot be transferred uncritically into the human
system as the murine and human immune systems are distinct from one another.
Our work group selectively examines human pDCs. Although human pDCs share
many similarities with their murine counterpart, there exist relevant differences:
3
Murine pDCs express CD11c on their surface, in the human system a marker for
classical DCs. Moreover murine pDCs are able to secrete IL-12 [6]. Besides, all
DC subsets in the murine immune system can respond to CpG DNA whereas only
B cells and pDCs respond to CpG molecules in the human system [76].
In conclusion, human pDCs represent a rare cell subset with diverse functions in
the immune system and display immunogenic as well as tolerogenic features.
1.2 Granzyme B- new roles and functions
The serine protease Granzyme B (GrB) is a granule enzyme that cleaves after
aspartate residues [156]. GrB has long been known as the major constituent of the
granules of NK cells and cytotoxic CD8+ T cells [31, 168]. The classical function of
GrB is the induction of cell death in target cells, like virally infected or tumor cells,
in a perforin-dependent manner via cleavage of caspase-3 [37, 123] or BH3
interacting domain death agonist (BID) [8, 70]. Besides, many different nonclassical sources of GrB have been discovered such as cytolytic CD4+ T cells [31,
150], B cells [69], hematopoietic progenitor cells [12], mast cells [163], basophils
[170], macrophages [94] and pDCs [87, 147].
Apart from the classical cell-death inducing function, it has been shown that GrB
can exert other immunogenic functions: Not only death effector molecules are
substrates of human GrB, GrB can also cleave and inactivate a wide range of
intracellular molecules that are important in cellular homeostasis which allows GrB
to interfere with viral replication (reviewed in [148]). Furthermore GrB can also
directly cleave essential viral proteins [148].
GrB also seems to exert diverse functions in an extracellular milieu. GrB is able to
cleave receptors important for cell survival such as Notch1 and Fibroblast growth
factor receptor 1 (FGFR1) [111] or receptors important for cell signaling and
interaction like glutamate receptor (GluR)3 on T cell receptor (TCR)-activated T
cells or the T cell receptor ζ-chain [177].
GrB might also modulate cell survival via modulation of the extracellular matrix,
thus inducing anoikis, programmed cell death as a result of the cleavage of
anchor-proteins of cells to the extracellular matrix, for example via cleaving of
laminin, fibronectin, vitronectin [22]. GrB can also cleave the cartilage
proteoglycan aggrecan [57]. In addition GrB was shown to increase vascular
4
permeability via release of vascular endothelial growth factor (VEGF) present in
the extracellular matrix [75].
It has been shown that GrB is regularly present in the plasma of healthy
individuals; in one study the median level of GrB was determined as 11.5 pg/ml
[161], whereas relevantly elevated extracellular levels of GrB have been detected
in several inflammatory states. In rheumatoid arthritis GrB is markedly elevated in
the synovial fluid of patients and to a lower extent in the plasma [161, 165]. In the
bronchoalveolar lavage of patients suffering from atopic asthma or chronic
obstructive pulmonary disease elevated GrB concentrations were measured [19,
77]. Not only in autoimmune diseases but also in responses to pathogens,
especially to viruses, extracellular GrB seems to play an important role. In blood
samples from patients infected with human immunodeficiency virus 1 (HIV-1) in an
asymptomatic phase and in serum of patients with acute infectious mononucleosis
GrB was elevated [161]. Also in dengue fever [23] and cytomegalovirus infection
after renal allograft transplantation [166], GrB was found to be increased.
In contrast to these rather immunogenic functions of GrB, GrB can exert
tolerogenic functions: Human adaptive regulatory T1 (Tr1) cells expressed GrB
and killed target cells in a perforin-dependent manner indicating a role of GrB in
immune system homeostasis [63]. In mice GrB could mediate a cell contactdependent tolerogenic mechanism of regulatory T cells (Tregs) in a perforinindependent way [61].
Previous work from our lab found that human pDC-derived GrB could inhibit T cell
proliferation [87].
1.3 Plasmacytoid dendritic cell-derived granzyme B
GrB expression in pDCs seems to be post-transcriptionally regulated; upon
activation pDCs produce fresh GrB [87, 147]. GrB secretion by pDCs requires
endosomal acidification and an active transport from the ER to the Golgi
apparatus. In addition GrB seems to be peri-nuclearly localized in granules of
pDCs and can be transferred to T cells in a perforin-independent but seemingly
cell contact-dependent manner. Astonishingly, pDCs secrete markedly higher
amounts of GrB than NK cells or CTLs. In mixed lymphocyte reactions (MLRs) our
lab has found that pDC-derived GrB could mediate suppression of both CD4+ and
5
CD8+ T cell proliferation in a perforin-independent manner [87]. The antiproliferative effect on CD4+ T cells could be mediated by cleavage of the T cell
receptor ζ-chain by pDC-derived GrB [47].
GrB production in pDCs is enhanced when pDCs are incubated with IL-3. Yet, the
combination of IL-3 and IL-10 was the most potent stimulus for GrB-induction in
pDCs. But even freshly isolated pDCs produce a small amount of GrB. When
pDCs are stimulated with a class B CpG ODN (CpG B), a stimulus that imitates
viral infection as it is an agonist of TLR9, GrB production is diminished and T cell
proliferation in MLRs is enhanced again [87]. Previous work tested the effect of
anti-viral vaccines containing ssRNA viruses on pDCs [47]. These anti-viral ssRNA
vaccines diminished GrB production by pDCs. Additionally, pDCs isolated from
patients who were recently vaccinated against tick-borne encephalitis virus
(TBEV), produced less GrB than pDCs from unvaccinated control patients. PDCs
treated with a TBEV vaccine did no longer transfer GrB to CD4+ T cells and T cell
proliferation was increased.
1.4 Clinical importance of viruses used in this study
Which role pDCs actually play in the anti-viral immune response is not clear.
Disease models in mice try to reveal the real importance of pDCs for anti-viral
immune responses in vivo. The authors of one review hypothesize that pDCs
might be essential in systemic viral infections and for infections with lymphotropic
viruses. Furthermore they suppose that pDCs might be relevant for the clearance
of infections that depend on strong CTL responses [164]. The human
herpesviruses varicella-zoster virus, cytomegalovirus and Epstein-Barr virus, but
also the retrovirus HIV-1 are all lymphotropic viruses and give rise to systemic
infections. For the immune response against these viruses, CTLs are particularly
important. Thus, in analogy to the supposed role of pDCs in murine anti-viral
immunity, the effects of these viruses on human blood pDCs are interesting to
evaluate as human pDCs are ascribed an important role in the immune response
against these viruses.
The human herpesviruses varicella-zoster virus, cytomegalovirus, and EpsteinBarr virus are dsDNA viruses that share relevant common features. A high
percentage of the human population is symptomless carrier of these viruses. After
6
primary infection herpesviruses can establish lifelong latency in the human host.
Reactivation can occur under certain circumstances such as inflammation and
above all immune suppression, for example caused by immunosuppressive
treatment after transplantation or HIV-1 infection. Of note, herpesviruses have
developed many mechanisms to co-exist with the host. They express a variety of
proteins to interact for example with antigen presentation or cell death [58, 83,
112, 113]. Importantly, herpesviruses, efficiently kept under control by a healthy
immune system, can cause relevant morbidity in the immunocompromised host.
The α-herpesvirus varicella-zoster virus (VZV) is a dsDNA virus. VZV is highly
contagious and is transmitted via droplet infection and enters respiratory epithelia
of the mouth from where virus is distributed in two phases of viremia to the
reticulo-endothelial system and thereafter to the skin. After an incubation period of
10 up to 21 days primary infection with VZV causes varicella. Varicella or
otherwise named chickenpox presents itself as a vesiculo-pustular ubiquitous
cutaneous rash accompanied by symptoms such as fever or lethargy. VZV
establishes latency in neurons of spinal ganglia and can therefore be reactivated
particularly in the elderly or in immunocompromised people. The reactivation
named herpes zoster or shingles usually presents itself as a localized painful
cutaneous efflorescence [32]. The commercially available VZV vaccines, one is
Varilrix®, introduced in 1984 [167], contain a live attenuated VZV strain derived
from a clinical isolate that is able to induce potent and protective immune
responses in vaccinated people [32].
Human cytomegalovirus (CMV) is a dsDNA virus that belongs to the subfamily of
β-herpesvirinae. In immunocompetent people primary CMV infection transmitted
by bodily secretions does seldom present clinical symptoms. Only in rare cases it
causes a febrile mononucleosis-like disease. A high percentage of the population
that increases with age is CMV positive without ever presenting symptoms [127].
Nevertheless, CMV infection is inducing a transient immunosuppression of a few
weeks to months [131]. After primary infection CMV establishes latency in
leukocytes. CMV infection usually causes problems in immunocompromised
individuals and in the course of vertical infections of fetuses inducing congenital
defects such as hearing loss, chorioretinitis and mental retardation among others.
Infection with CMV or its reactivation can cause major problems after
transplantations or during other situations of a compromised immune system like
7
in acquired immunodeficiency syndrome (AIDS) where CMV may arise as an
opportunistic infection for example as retinitis or pneumonitis [127].
Epstein-Barr virus (EBV), a dsDNA γ-herpesvirus, is transmitted orally and causes
mononucleosis, the kissing disease, mainly in young people which goes along with
generalized
lymphadenopathy,
fatigue
and
hepatosplenomegalie.
A
high
percentage of the population is asymptomatic carrier of EBV as EBV establishes
latency in B cells. EBV causes highly relevant problems in immunocompromised
patients such as transplant receivers or patients suffering from AIDS as posttransplant lymphoproliferative disease and B cell lymphomas can occur. EBV is
oncogenic and consequently a potent promotor of diverse cancers such as
Burkitt’s lymphoma or nasopharyngeal carcinoma [146].
The (+) ssRNA retrovirus HIV-1 was discovered in 1984 and causes AIDS. The
HIV epidemic represents a major global health problem. HIV-1 transmission is
possible in a parenteral, sexual or vertical way. Primary infection with HIV-1
usually presents like an acute viral infection but is often associated with
lymphadenopathy and weight loss. An asymptomatic phase follows with ongoing
CD4+ T cell loss ultimately leading to the establishment of AIDS after a varying
time span with the onset of opportunistic infections or the manifestation of AIDSdefining diseases. PDCs are known to play an important role during HIV-1
infection and it still remains to be elucidated whether their role is rather beneficial
or more detrimental for the spread and course of the disease [98].
8
1.5 Hypothesis and aims
As pDCs are important mediators between innate and adaptive immunity
especially in the course of viral infections, they are decisive for the induction of a
potent immune response against viruses and in the establishment of immunity
upon immunization with anti-viral vaccines. Recently it was shown that anti-viral
vaccines against ssRNA viruses modulate GrB expression by pDCs [47].
Therefore in this thesis, the effects of an anti-viral vaccine against a dsDNA virus,
the varicella-zoster virus (VZV) vaccine Varilix® consisting of a live attenuated
virus strain, were assessed. Then, to go beyond the model of anti-viral vaccines,
we analyzed two other dsDNA herpesviruses: Ultraviolet light (UV)-inactivated
human cytomegalovirus (CMV) and Epstein-Barr virus (EBV) against which no
vaccines have been developed so far.
Due to the high pathogenicity of human immunodeficiency virus 1 (HIV-1) and the
interesting role pDCs seem to play in the course of the disease, we also tested the
effect of the ssRNA retrovirus HIV-1 on pDCs.
We hypothesized that GrB in pDCs is differentially and specifically regulated by
these viruses. The inhibition of GrB may contribute to an efficient anti-viral immune
response whereas increased GrB levels might dampen the initiation of a potent T
cell effector response. Thus GrB might be an additional variable affecting the
capacity of pDCs to either hamper or trigger adaptive immune responses in the
course of viral infections. We presumed that the vaccine-derived virus and the
other three viruses must also have a distinct effect on the immunophenotype of
pDCs.
In order to test this hypothesis, we asked the following questions:
•
Is GrB production and secretion by pDCs differentially regulated by the viral
agents tested?
•
Is the secretion of IFN-α, the major cytokine produced by pDCs, induced in
pDCs upon stimulation with the viruses tested?
•
Do
the
viruses
tested
influence
the
maturation
status
and
the
immunophenotype of pDCs assessed via surface molecule expression?
•
Do virus-stimulated GrB-producing pDCs have distinct effects on the
proliferation of CD4+ T cells in mixed lymphocyte reactions (MLRs)?
9
2 Material and Methods
2.1 Samples
The present study was approved by the Ethics Committee at Ulm University. In
order to isolate specific cell subsets buffy coats were obtained from the German
Red Cross in Ulm or 50 up to 250 ml of fresh peripheral blood was taken from
healthy volunteers after obtaining informed consent from each individual.
2.2 Buffers
2.2.1 Ammonium chloride potassium lysing buffer
For 1 l ammonium chloride potassium (ACK) lysing buffer 0.15 M ammonium
chloride (NH4Cl) (Merck KGaA, Darmstadt, Germany), 10.0 mM potassium
bicarbonate
(KHCO3)
(Sigma-Aldrich,
St.
Louis,
MO,
USA),
0.1
mM
ethylenediaminetetraacetic acid (EDTA) (Carl Roth, Karlsruhe, Germany) and 800
ml Ampuwa® Spüllösung Plastipur® Aqua ad iniectabilia (Fresenius Kabi France,
Sèvres, France) were mixed and adjusted to a pH between 7.2 and 7.4 with
sodium hydroxide (NaOH) (Sigma-Aldrich).
Then Ampuwa® Spüllösung Plastipur® Aqua ad iniectabilia was added to a final
volume of 1 l and the buffer was filtered sterile with a Vacuum Filtration System
(PES, 0.2 µm, 1 l) (VWR International, Radnor, PA, USA) and stored at room
temperature (RT).
2.2.2 Magnetic activated cell sorting buffer
To prepare magnetic activated cell sorting (MACS) buffer 0.5% Albumin bovine
Fraction V, pH 7.0 (bovine serum albumin (BSA)) (SERVA Electrophoresis GmbH,
Heidelberg, Germany) and 2 mM EDTA were added to 1 l of phosphate buffered
saline without calcium and magnesium (PBS) (Biochrom AG, Berlin, Germany).
After mixing ingredients, pH was adjusted to a value between 7.2 and 7.4 with
NaOH. Then buffer was filtered sterile, degassed and stored at 4 °C.
10
2.2.3 Fixation buffer
The paraformaldehyde (PFA) (Merck KGaA) stock was diluted in PBS to a final
concentration of 4%, filtered sterile and stored at 4 °C.
For HIV-1 and EBV experiments a 1:1 dilution of the fixation buffer with PBS was
used to be able to use the double amount of volume per tube.
2.2.4 Permeabilization buffer
For 100 ml permeabilization buffer 100 ml of PBS was mixed with 0.25% Saponin
(Sigma-Aldrich), filtered sterile and stored at 4 °C.
2.2.5 Roswell Park Memorial Institute medium
Roswell Park Memorial Institute (RPMI) 1640 medium (Life Technologies,
Carlsbad, CA, USA) was supplemented with 10% foetal bovine serum (FBS) (Life
Technologies), 2 mM L-glutamine (Life Technologies), 1 mM sodium pyruvate
(Biochrom AG) and
10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
(HEPES) buffer (Biochrom AG), which will be referred to as completed RPMI.
2.3 Viral stimuli
2.3.1 Varicella-zoster virus
As a source of varicella-zoster virus (VZV) we used the VZV vaccine Varilrix®
(GlaxoSmithKline Biologicals s.a., Rixensart, Belgium) that contains the live
attenuated varicella-zoster virus strain OKA, a clinical isolate that was propagated
in cultures of human diploid cells, the cell line MRC-5 that consists of human fetal
lung fibroblasts. Further ingredients of the lyophilized vaccine are amino acids,
human albumin, lactose, mannitol, sorbitol, phenol red, traces of neomycin sulfate,
traces of remaining stock from the cell culture and the culture media such as salts,
vitamins, sugar and BSA.
The lyophilized vaccine was reconstituted in 0.5 ml water for injection that was
included in the commercially available vaccine. 0.5 ml of the reconstituted vaccine
11
contains at least 103.3 plaque forming units. The whole reconstituted vaccine was
used in volume dilutions as follows: 1:2000, 1:200, 1:20 and 1:10. To test whether
the viral component in the varicella-zoster virus vaccine alone and no other
ingredients of the vaccine were responsible for the effects on pDCs, the
reconstituted vaccine was filtrated through a virus filter that allowed the removal of
viral particles. A PALL® Acrodisc® 32 mm Syringe Filter with a 0.1 µm Supor®
Membrane (Pall Corporation, Port Washington, NY, USA) that allowed only
particles smaller than 0.1 µm to pass through was used. The varicella-zoster virus
virion itself has a diameter of 180-200 nm [32]. Consequently, the filtrated
varicella-zoster virus vaccine should contain no more virions.
We also tested the effect of ultraviolet light (UV)-irradiated VZV vaccine on pDCs.
The reconstituted vaccine was inactivated via UV-irradiation in a UV CrossLinker
(CL-1000; UVP, Upland, CA).
2.3.2 Cytomegalovirus
Human cytomegalovirus (CMV) was kindly provided by Dr. Giada Frascaroli,
Institute of Virology, head Prof. Dr. med. Thomas Mertens, University Medical
Center Ulm. The endotheliotropic CMV strain TB40E was produced using human
foreskin fibroblasts (HFFs). Virus was inactivated via UV-irradiation with a UV
CrossLinker (CL-1000; UVP, Upland, CA) at a wavelength of 366 nm for 2 times 2
min what corresponded to an energy of 200 kJ. Virus aliquots were stored at -80
°C. We used only UV-inactivated CMV in our experiments. For easier labeling we
use the abbreviation CMV in the results section. In the discussion UV-inactivated
CMV is refered to as UV-CMV to distinguish the inactivated virus from active CMV.
As a control we were provided with ultra-centrifuged supernatant from mockinfected HFFs.
2.3.3 Epstein–Barr virus
Epstein-Barr virus (EBV) was propagated on B95-8 cells, an EBV-producing B cell
line derived from a tamarin (Saguinus oedipus) [125, 126], in RPMI medium (PAA
The Cell Culture Company, Pasching, Austria) supplemented with 10% FCS (PAA
The Cell Culture Company) and 1% penicillin/streptomycin (PAA The Cell Culture
12
Company).
After 21 days, cells were spun down and supernatant containing EBV in RPMI
medium was stored at either -30 °C or -80 °C.
To test whether the viral component in the EBV stock and no other ingredients in
the supernatant of the B95-8 cells were responsible for the effects on pDCs, the
EBV stock was filtrated through a virus filter that allowed the removal of viral
particles: A PALL® Acrodisc® 32 mm Syringe Filter with a 0.1 µm Supor®
Membrane (Pall Corporation, Port Washington, NY, USA) that allowed only
particles smaller than 0.1 µm to pass through. The EBV virion itself has a diameter
of approximately 115 nm [133]. Consequently, the filtrated EBV stock should
contain no more virions.
2.3.4 Human immunodeficiency virus 1
The human immunodeficiency virus (HIV) strain HIV-1_M_NL_43_wt was kindly
provided by Dr. Ali Gawanbacht, Institute of Molecular Virology, head Prof. Dr.
Frank Kirchhoff, Ulm University.
Virus stock was generated by transient transfection of HEK293T cells (ATCC,
Manassas, VA, USA), human embryonic kidney cells that are transformed with
Adenovirus Type 5 and express simian virus 40 (SV40) large T-antigen [62], with
the calcium chloride precipitation method.
Mock transfection of HEK293T cells was performed as a control. The only
difference to the protocol for virus generation was that no DNA was added during
the transfection of HEK293T cells. All other steps in mock generation were
performed as for virus generation.
2.4 Isolation of specific cell subsets
2.4.1 Isolation of peripheral blood mononuclear cells
In order to isolate peripheral blood mononuclear cells (PBMCs) from buffy coats or
fresh blood, blood was diluted 1:2 with PBS (Biochrom AG). Up to 35 ml of diluted
blood were layered on 15 ml of Biocoll separating solution (Biochrom AG) in a 50
ml polypropylene conical tube (Becton Dickinson, Franklin Lakes, NJ, USA) for
density gradient centrifugation.
13
Tubes were filled up to 50 ml with PBS and were spun at 1000 g for 15 min at RT
with brakes off for density gradient formation. Most of the erythrocytes and
granulocytes are to be found at the bottom of the tube because of their higher
density. Beneath the blood plasma on top, mononuclear blood cells or so called
peripheral blood mononuclear cells (PBMCs) form a thin white ring that was
harvested in 2 fresh 50 ml tubes. Then the tubes were filled up to 50 ml with PBS.
After centrifuging the tubes at 300 g for 10 min at RT, supernatant was discarded;
cell pellets were re-suspended and unified in one tube per donor. Cells were
washed again with PBS in 50 ml volume (200 g, 15 min, RT).
After centrifugation supernatant was discarded, the cell pellet re-suspended and 7
ml of ACK lysing buffer were added per tube and mixed with the cells to lyse
erythrocytes. After 7 min of incubation at RT, tubes were filled up with PBS to 50
ml and cells were washed (300 g, 10 min, RT). Supernatant was discarded and
the cell pellet re-suspended. After filling up the tube to exactly 50 ml about 150 µl
of cell suspension per donor were taken to count the cells and to analyze the
percentage of pDCs per donor via flow cytometry, if pDC isolation was the final
aim.
For cell count 10 µl of a mix of 5 µl cell suspension and 95 µl trypan blue (SigmaAldrich) were administered into a Neubauer counting chamber (Laboroptik,
Friedrichsdorf, Germany). Cells were counted at a 10-fold magnification with a
CK30 culture microscope (Olympus Corporation, Tokyo, Japan). Cell numbers
were determined with the formula: Sum of 4 cell counts/4 x 20 (dilution factor with
trypan blue) x 104 (0.1 µl volume per cell count field)/ml.
The tubes containing the cell suspension were centrifuged (200 g, 15 min, RT),
supernatant was discarded and the cell pellet was re-suspended. Then cells were
centrifuged (300 g, 10 min, RT) in 20 ml of MACS buffer to adapt PBMCs to the
new buffer milieu used for further cell subset isolation via MACS cell separation
reagents (Miltenyi Biotec GmbH, Bergisch-Gladbach, Germany).
2.4.2 Isolation of plasmacytoid dendritic cells
To assess whether the isolated PBMCs contained enough pDCs per donor to
continue isolation, a flow cytometry analysis was performed. 100 µl of cell
suspension of PBMCs at the counting step were stained with 3 µl of a
14
phycoerythrin (PE)-conjugated BDCA-2 monoclonal antibody (mAb) (Miltenyi
Biotec GmbH) and 1 µl of a fluorescein isothiocyanate (FITC)-conjugated lineage
cocktail 1 (lin 1) (Becton Dickinson) per tube for 15 min in the dark. The lin 1
cocktail consists of mAbs against the following molecules: Cluster of differentiation
(CD)3, CD14, CD19, CD20, CD56. After a washing step (2 ml PBS per tube, 350
g, 7 min, RT) cells were analyzed with a FACS Scan flow cytometer (Becton
Dickinson) for viability and percentage of pDCs. PDCs were defined as BDCA-2+,
lin 1– cells.
2.4.3 Positive selection of plasmacytoid dendritic cells
PDCs were positively selected from PBMCs with a CD304 (BDCA-4/Neuropilin-1)
MicroBead Kit human (Miltenyi Biotec GmbH) according to the manufacturer’s
protocol.
The PBMC pellet adapted to MACS buffer during the last washing step was resuspended in 150 µl MACS buffer per 108 cells. Then 50 µl of FcR Blocking
reagent per 108 cells and 50 µl of CD304 MicroBeads per 108 cells were added
and cells were incubated for 15 min at 4 °C in the dark.
Cells were washed with 20 ml MACS buffer (300 g, 10 min, RT), supernatant was
discarded and the pellet re-suspended in 500 µl MACS buffer per 108 cells.
One LS column (Miltenyi Biotec GmbH) per donor was put into the quadroMACS™
Separation Unit (Miltenyi Biotec GmbH) and moistened with 3 ml MACS buffer.
Then the cell suspension was applied to the columns. After letting the whole cell
suspension run through the column, the columns were washed three times with 3
ml MACS buffer, adding the new volume only after the previous one had gone
through the column. Then 5 ml MACS buffer were added to each column and
magnetically labeled pDCs retained in the column were flushed out by firmly
pushing the plunger into the upper part of the column.
The eluted suspension, containing pDCs, was once again applied to a rinsed LS
column to increase purity. This second column was again three times washed with
3 ml MACS buffer and pDCs retained in the column were flushed out into a 15 ml
polypropylene conical tube (Becton Dickinson) by firmly squeezing 5 ml of MACS
buffer through the column with a plunger.
Cell count was performed with a Neubauer counting chamber (10 µl of a mix of 10
15
µl cell suspension and 10 µl trypan blue).
Flow cytometry analysis of 100 µl cell suspension was performed as described
above. Viability and purity of pDCs, defined as percentage of viable BDCA-2+, lin
1– cells, were assessed. Median purity after positive selection of pDCs was > 90%
BDCA2+, lin 1- cells. Median viability was > 48%.
The cell suspension of purified pDCs was spun down (300 g, 10 min, RT) and the
cell pellet re-suspended in Adoptive Immunotherapy Media (AIM-V) (Life
Technologies) or in completed RPMI in order to start cell culture.
2.4.4 Negative selection of plasmacytoid dendritic cells
For negative selection of pDCs from PBMCs the Plasmacytoid Dendritic Cell
Isolation Kit human (Miltenyi Biotec GmbH) was used according to the
manufacturer’s protocol.
The PBMC pellet, adapted to MACS buffer during the last slow washing step, was
re-suspended in 200 µl MACS buffer per 108 cells. 50 µl per 108 cells of a BiotinAntibody Cocktail were added to and mixed with the cells. The Antibody cocktail
contained Abs against molecules expressed on cells such as myeloid DCs,
monocytes, T cells, B cells, NK cells, granulocytes and erythroid cells in order to
label cells other than pDCs.
After an incubation time of 10 min at 4 °C in the dark, cells were two times washed
with 5-10 ml MACS buffer per 108 cells (300 g, 10 min, RT).
Then the cell pellet was re-suspended in 200 µl MACS buffer per 108 cells and 50
µl Anti-Biotin MicroBeads per 108 cells were added and mixed with the cells. Cells
were incubated for 15 min in the dark at 4 °C. After a washing step with 5-10 ml
MACS buffer per 108 cells (300 g, 10 min, RT) the pellet was re-suspended in 500
µl MACS buffer per 108 cells.
LD columns (Miltenyi Biotec GmbH) were put into the quadroMACS™ Separation
Unit and moistened with 2 ml MACS buffer. 108 cells were applied per LD column
and columns were washed twice with 2 ml MACS buffer.
Flow-through containing pDCs was collected in 15 ml tubes.
PDCs were counted in a Neubauer counting chamber (10 µl of a mixture of 10 µl
cell suspension and 10 µl trypan blue) and a viability and purity flow cytometry
16
analysis was performed like described above. Median purity of BDCA2+, lin 1- cells
after negative selection was > 89%, median viability was > 86%.
The cell suspension was spun down (300 g, 10 min, RT) and cells were resuspended in AIM-V or completed RPMI medium.
2.4.5 Negative selection of CD4+ T cells
In order to isolate CD4+ T cells from PBMCs the CD4+ T Cell Isolation Kit II human
(Miltenyi Biotec GmbH) was applied according to manufacturer’s protocol.
PBMCs adapted to MACS buffer were re-suspended in 40 µl MACS buffer per 107
cells. 10 µl per 107 cells of the Biotin-Antibody Cocktail containing Abs against
CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCRγ/δ and Glycophorin A were
added and the mixture was incubated for 10 min at 4 °C in the dark. Then 30 µl of
MACS buffer per 107 cells and 20 µl of Anti-Biotin MicroBeads per 107 cells were
added and the mixture was re-incubated at 4 °C for 15 min in the dark.
The cell suspension was then washed with 20 ml MACS buffer (300 g, 10 min, RT)
and up to 108 cells were re-suspended in 500 µl MACS buffer.
For magnetic separation of CD4+ T cells LS columns were put into a
quadroMACS™ Separation Unit and moistened with 3 ml MACS buffer. The cell
suspension was applied to the column and the column rinsed three times with
MACS. Flow-through containing purified CD4+ T cells was collected in 15 ml tubes.
Cells were counted in a Neubauer counting chamber and viability and purity of
cells was assessed via flow cytometry after staining the cells like described above
with a CD3-PE mAb (Becton Dickinson). Median purity of T cells after negative
selection was > 95% CD3+ cells, median viability was > 95%.
Cells were spun down (300 g, 10 min, RT) and re-suspended in AIM-V or
completed RPMI medium or were stained with carboxyfluorescein diacetate
succinimidyl ester (CFSE) (CellTrace™ CFSE Proliferation Kit; Life Technologies)
as described in the section mixed lymphocyte reaction (MLR).
17
2.5 Cell culture
2.5.1 Short term cell culture
For analysis of Granzyme B (GrB) production and GrB and IFN-α secretion pDCs
were incubated in AIM-V directly after positive selection. PDCs were seeded at a
density of 5 x 105 cells/ml, 200 µl/well in 96-well flat-bottom plates (Becton
Dickinson). For most experiments, pDCs were incubated in medium supplemented
with IL-3 at a final concentration of 10 ng/ml. For experiments with HIV-1 the
cytokine IL-10 was applied at a final concentration of 25 ng/ml. PDCs were
incubated for 16 h at 37 °C and 5% carbon dioxide (CO2) in a WTB Binder
incubator (Binder GmbH, Tuttlingen, Germany). PDCs for experiments with EBV
were incubated 1:1 in AIM-V and RPMI supplemented with 10% FCS and 1%
penicillin/streptomycin, since the EBV stock was based on RPMI supplemented
with 10% FCS and 1% penicillin/streptomycin. After 16 h of incubation, pDCs were
stained for intracellular GrB and surface molecules and supernatant was analyzed
for GrB and IFN-α secretion.
2.5.2 Mixed lymphocyte reactions
PDCs for mixed lymphocyte reactions (MLRs) were negatively selected and then
incubated for 24 or 48 h in IL-3-supplemented AIM-V medium (10 ng/ml) in the
presence of different stimuli at a density of 5 x 105 cells/ml, 200 µl/well in a WTB
Incubator at 37 °C and 5% CO2.
To prepare pDCs for MLRs, first 150 µl of supernatant were harvested, 150 µl of
fresh completed RPMI were added to the cells and then pDCs were harvested in 2
ml safe-lock tubes (Eppendorf AG, Hamburg, Germany). Wells were washed two
times with 200 µl completed RPMI to harvest all cells. PDCs were washed at 270
g, RT, for 5 min using a 5417C centrifuge (Eppendorf AG). Supernatant was
discarded, the cell pellet was re-suspended in 1 ml completed RPMI and cells
were counted with a Neubauer chamber and trypan blue. Cells were washed again
(270 g, RT, 5 min). Then pDCs were re-suspended in completed RPMI.
CD4+ T cells were negatively selected on day one or two. After isolation CD4+ T
cells were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE).
18
T cells were re-suspended in 380 µl PBS per 1 x 107 cells, CFSE at a final
concentration of 2.5 µM was added, properly mixed and the whole tube content
was transferred into a new one. Cells were incubated for 10 min at 37 °C and 5%
CO2. Then 10 ml completed RPMI were added to block the staining reaction during
another 10 min at RT.
Afterwards tubes were filled up to 50 ml with PBS and washed twice (300 g, 10
min, RT). Washing included discarding of supernatant and re-suspension of cells
in fresh PBS. Cells were counted in a Neubauer chamber with trypan blue
staining.
The third washing step was performed with completed RPMI medium. Then cells
were re-suspended in completed RPMI.
Proper and equal staining of CD4+ T cells with CFSE was evaluated via flow
cytometry with a FACS Scan flow cytometer.
PDCs and CD4+ T cells were seeded at different ratios in 96-well round-bottom
wells (Becton Dickinson). 2 x 105 CD4+ T cells were seeded with pre-treated
allogeneic pDCs at the ratios 1:50, 1:250 and 1:1250 in 200 µl completed RPMI.
Cells were co-incubated for six or seven days and then harvested into FACS tubes
for staining with a PE-conjugated CD3 mAb to label T cells and shortly before
analysis with a LSR II (Becton Dickinson) cells were also stained with 7-aminoactinomycin D (7-AAD) (Merck KgaA, Darmstadt, Germany) to exclude dead cells.
Staining for flow cytometry is described in detail in the section flow cytometry.
Proliferated CD4+ T cells were defined as CD3+, 7-AAD-, CFSElow cells for
experiments with VZV and CMV. For experiments with EBV and HIV-1 proliferated
CD4+ T cells were defined as CD3+, CFSElow cells. PDCs die during a cell culture
time of six or seven days.
As controls 2 x 105 CD4+ T cells were incubated with 0.25 or 0.5 µl CD3/CD28
beads (Dynabeads® CD3/CD28 T cell Expander, Dynal Biotech ASA, Oslo,
Norway) or in 200 µl completed RPMI alone.
As a control for potent pDC stimulation for allogeneic induction of T cell
proliferation we incubated pDCs with a class B cytosine-phosphate-guanine
oligodeoxynucleotide (CpG B) with the sequence 5’-tcg tcg ttt tgt cgt ttt gtc gtt-3’
(ODN 2006; Invivogen, San Diego, CA, USA). PDCs incubated with 2.5 µg/ml CpG
B produce less GrB than pDCs incubated in IL-3-supplemented medium alone and
induce T cell proliferation in MLRs [87].
19
2.5.3 Mixed lymphocyte reactions with HIV-1-infected CD4+ T cells
CD4+ T cells were isolated as described above. 3 x 106 T cells were re-suspended
in 1 ml supplemented RPMI medium (Life Technologies) (10% heat-inactivated
FCS, 350 µg/ml L-glutamine, 120 µg/ml penicillin, 120 µg/ml streptomycin) and
transferred into a 50 ml cell culture flask for suspension cells (Sarstedt AG & Co.,
Nümbrecht, Germany).
T cells were stimulated with 25 µl CD3/28 beads (Dynabeads® Human T-Activator
CD3/CD28, Life Technologies) per 1 x 106 T cells.
Beads were prepared by washing them once with at least 1 ml PBS (PAA
Laboratories GmbH, Pasching, Austria/Life Technologies). Volume of PBS should
be at least the volume of beads used. A 5 ml polystyrene round-bottom tube
(Becton Dickinson) containing beads in PBS was vortexed and then put for 1 min
in a DynaMag™ magnet (Life Technologies). PBS was discarded and the tube
was filled with supplemented RPMI and added to T cells in the flask. Finally, 2 x
106 T cells were re-suspended in 1 ml supplemented RPMI each and incubated for
three days at 37 °C, 5% CO2 (STERI cult, Thermo Fisher Scientific, Waltham, MA,
USA) in a 50 ml cell culture flask for suspension cells (Sarstedt AG & Co.). At day
one (only in one experiment) or yet at d0 10 ng/ml IL-2 (Miltenyi Biotek GmbH)
were added to the medium.
After three days of stimulation, beads were removed from the cells with a
DynaMag™ magnet. Therefore, content of the cell culture flask was transferred
into a 15 ml tube (Sarstedt AG & Co.), the tube was vortexed and put into a
DynaMag™ magnet for 1 min, fluid was transferred into a new tube whilst beads
stayed attached to the walls of the tube. 1 x 106 T cells were put each into 5 ml
tubes.
After centrifugation (340 g, 3 min, RT) supernatant was discarded and 300 µl virus
stock were added to 1 x 106 T cells or centrifuged supernatant of mock-transfected
HEK293T cells was added as a control. Cells were transduced for 6 h at 37 °C,
5% CO2 (HERAcell® 240, Thermo Fisher Scientific). Cells were washed twice in
PBS, then 3 ml supplemented RPMI plus 10 ng/ml IL-2 (Miltenyi Biotec GmbH)
were added per tube. Tubes were vortexed, content was transferred into 6-well
plates (Greiner Bio-One, Frickenhausen, Germany) and incubated at 37 °C, 5%
CO2 for three days.
20
On day seven, pDCs were negatively selected as described above and stimulated
with IL-3 (10 ng/ml), IL-10 (25 ng/ml) or a combination of IL-3 and IL-10 (IL-3 + IL10). PDCs were co-incubated with HIV-infected or mock-infected T cells in a ratio
of 1 x 105 pDCs: 2 x 105 T cells in 200 µl supplemented RPMI in a 96-well flatbottom plate (Greiner Bio-One).
At day eight, 150 µl supernatant were collected and stored at -20 °C for enzymelinked immunosorbent assay (ELISA) analysis.
2.6 Enzyme-linked immunosorbent assay
75 µl supernatant from pDCs incubated for 16 or 48 h were taken in order to
measure GrB or IFN-α in the supernatant. Supernatant was usually stored at –80
°C for experiments with VZV and CMV, at -20 °C or -40 °C for EBV and at -20 °C
for HIV experiments.
2.6.1 Granzyme B ELISA
Plates were assembled using Nunc-Immuno™ Modules (Thermo Fisher Scientific,
Waltham, MA, USA).
For analyzing GrB in supernatant the Human Granzyme B ELISA kit (Mabtech AB,
Nacka Strand, Sweden) was applied according to the manufacturer’s protocol.
One day before ELISA the plate was covered with 100 µl/well of a GrB–Coating
Antibody (mAb GB10) that was diluted to a final concentration of 2 µg/ml with PBS.
The plate was covered with Parafilm “M” Laboratory Film (Pechiney Plastic
Packaging, Menasha, WI, USA) and stored overnight in a humid chamber at 4 °C.
The next day the Ab-covered plate was washed twice with 200 µl/well PBS and
subsequently incubated for 1 h with 200 µl/well of incubation buffer to block
unspecific binding (PBS with 0.05% Tween 20 (Sigma-Aldrich) and 0.1% BSA).
GrB standards were prepared with incubation buffer ranging from 4000 pg/ml to
62.5 pg/ml in dilution steps of 1:2. Following five washing steps with 200 µl/well
PBS, 100 µl/well of samples diluted with incubation buffer and GrB standards were
added to the plate; incubation buffer was used as blank. The plate was covered
with a plate sealer (Human IFN-α Multi-Subtype ELISA Kit; PBL Biomedical
Laboratories, Piscataway, NJ, USA) and incubated for 2 h at RT in the dark. Plate
21
was washed five times. Then 100 µl/well of a Biotin tagged GrB-Detection Ab
(mAb GB11-biotin) diluted in incubation buffer to a final concentration of 1 µg/ml
were added. After 1 h of incubation and five washing steps 100 µl/well of
Streptavidin-HRP 1:1000 diluted with incubation buffer were added and incubated
for 1 h at RT. Finally a 1:1 mixture of colour reagent a (stabilized peroxide
solution) and colour reagent b (stabilized chromogen solution) from a Substrate
Reagent Pack (R&D Systems, Minneapolis, MN, USA) was added and incubated
for 20 min in the dark without covering. Color reaction was stopped with 50 µl/well
of 2N sulphuric acid (Sigma-Aldrich). ELISA was measured immediately at a
wavelength of 450 nm with a Mithras LB 940 micro-titer plate reader (Berthold
Technologies GmbH & Co. KG, Bad Wildbad, Germany) for VZV and most CMV
samples, whereas EBV, HIV-1 and some CMV samples were measured with a
Thermomax® microplate reader (Molecular Devices, LLC., Sunnyvale, CA, USA).
2.6.2 Interferon-α ELISA
For measuring of IFN-α in the supernatant of pDCs the Human IFN-α MultiSubtype ELISA Kit (PBL Biomedical Laboratories, Piscataway, NJ, USA) was used
according to the manufacturer’s protocol.
Standards were prepared by diluting Human IFN Alpha standard (10,000 pg/ml)
with dilution buffer. Extended range ELISA IFN-α standards covered a range
between 5000 pg/ml and 78.125 pg/ml in steps of 1:2 dilutions. High sensitivity
IFN-α ELISA standards ranged between 500 pg/ml and 6.25 pg/ml and were
prepared by 1:2 dilutions from 200 pg/ml down to 6.25 pg/ml.
100 µl of samples diluted with dilution buffer, standards and dilution buffer as blank
were added to the pre-coated IFN-α ELISA plate. Plate was covered with a plate
sealer and incubated for 1 h in the dark. After washing the plate twice with 250
µl/well wash buffer (Wash solution concentrate 1:20 diluted in Ampuwa®
Spüllösung Plastipur® Aqua ad iniectabilia), 100 µl/well of diluted antibody solution
(Antibody concentrate diluted in dilution buffer according to the lot-specific
certificate of analysis) was added and incubated for 1 h in the dark.
Three washing steps (250 µl/well wash buffer) were followed by addition of 100
µl/well of diluted HRP solution (HRP conjugate concentrate diluted in HRP
22
conjugate diluent according to the lot-specific certificate of analysis). Plate was
covered and incubated for 1 h.
The
plate
was
washed
four
times
(250
µl/well
wash
buffer)
and
a
Tetramethylbenzidine (TMB) substrate, warmed to room temperature, was added
(100 µl/well) and incubated for 15 min in the dark without covering. After adding 50
µl/well of stop solution color reaction was measured immediately at a wavelength
of 450 nm with a Mithras LB 940 micro-titer plate reader in the case of most VZV
and some CMV samples or in case of EBV, HIV and some CMV and VZV samples
with a Thermomax® microplate reader.
As a control for potent IFN-α induction in pDCs we used 1 µg/ml of a class A
cytosine-phosphate-guanine oligodeoxynucleotide (CpG A) with the sequence 5’g*g*g gac gac gtc gtg g*g*g * g*g*g-3’ (*phosphorothioate bonds, rest are
phosphodiester bonds) (ODN 2336; Coley Pharmaceutical Group, Ottawa, ON,
Canada).
2.7 Flow cytometry
2.7.1 Surface staining
PDCs intended to be stained for intracellular GrB were incubated for 4 h with
1 µg/ml Brefeldin A (Epicentre Biotechnologies, Madison, WI, USA) prior to
harvesting. Staining for surface molecules was performed before intracellular
staining for GrB. Other pDCs and T cells were only stained for surface molecules.
For surface staining, cells were harvested and 3 µl of the respective mAb were
added, cells were vortexed and incubated for 15 min in the dark. Cells were
washed (2 ml PBS, 350 g, RT, 7 min), supernatant aspirated except for 200 µl and
cells were analyzed with a flow cytometer.
MAbs against the following surface molecules were used: CD3, CD14, CD16,
CD19, CD20, CD40, CD54, CD56, CD80, CD83, CD86, CD123, HLA-ABC and
HLA-DR (all from Becton Dickinson).
The mAbs were conjugated with different fluorescent dyes: Allophycocyanin
(APC), FITC, PE, phycoerythrin tandem with cyanin dye 5 (PE-Cy5) and peridinin
chlorophyll protein tandem with cyanin dye 5.5 (PerCP-Cy5.5).
23
2.7.2 Staining for intracellular granzyme B
After 16 h of incubation of pDCs at 37 °C, 5% CO2, 150 µl of supernatant were
taken for analysis with ELISAs. This volume was re-substituted. In case of
experiments intended for the analysis of the effect of VZV on pDCs, 75% of the
initial amount of the incubation reagents medium, IL-3 and VZV volume dilutions
was re-substituted. In case of experiments with CMV, EBV and HIV-1 no virus or
cytokines, only medium was re-substituted.
After re-substitution of volume into the wells, cells were incubated for 4 h at 37 °C,
5% CO2 with 1 µg/ml Brefeldin A (Epicentre Biotechnologies, Madison, WI, USA).
Samples for the analysis of the basic GrB-production level in freshly isolated
pDCs, named d0 samples, were incubated with Brefeldin A for 4 h directly after
isolation. Brefeldin A is a reagent that blocks the transport of proteins from the ER
to the Golgi apparatus. Therefore intracellular GrB accumulated in the ER.
Then pDCs were harvested in 5 ml polystyrene round-bottom tubes (Becton
Dickinson) and were stained for surface molecules. 3 µl per mAb were used per
tube, except for lin 1-FITC where 1 µl per tube was used. Cells were vortexed and
incubated for 15 min in the dark. Afterwards cells were washed with 2 ml PBS at
350 g for 7 min at RT. Supernatant was aspirated and the cell pellet with about
100 µl of volume was left in the tube. Then cells were fixed for 15 min in 100 µl of
fixation buffer. In case of incubations with HIV or EBV, 200 µl of a 2% PFA solution
were used.
After another washing step (2 ml PBS, 350 g, RT, 7 min), supernatant was
aspirated and 100 µl of permeabilization buffer were added per tube. At once 2 µl
of FcR Blocking reagent human (Miltenyi Biotec GmbH) were added, cells were
vortexed and incubated for 10 min in the dark.
Then 2 µl of a mAb for GrB (Sanquin Blood Supply, Amsterdam, The Netherlands)
that was conjugated with PE or the isotype control Ab (Mouse immunoglobulin
(Ig)G1 isotype control PE-conjugated; R&D Systems, Minneapolis, MN, USA) were
added, tubes were vortexed and cells incubated for another 15 min in the dark.
Cells were washed and 200 µl volume per tube was left after aspiration of
supernatant to have enough volume left to analyze cells with a flow cytometer,
either a FACS Scan, a FACS Calibur or a LSR II (Becton Dickinson).
24
2.7.3 Compensation controls
For compensation, PBMCs left from the pDC isolations were incubated overnight
and were stained for CD45 conjugated to the respective dyes according to the
staining protocol for the pDCs intended for the same readout; if pDCs were fixed
and permeabilized, PBMCs, used for compensation, were first stained for CD45
and then fixed and permeabilized as well. All CD45 Abs, conjugated with the
fluorescent dyes APC, FITC, PE, PE-Cy5, PerCP-Cy5.5, used for staining of
compensation controls were provided by Becton Dickinson.
2.7.4 Staining with 7-amino-actinomycin D
To be able to exclude dead cells during flow cytometric analysis of MLRs, 3 µg/ml
of 7-amino-actinomycin D (7-AAD) were added to the cells shortly before
measurement.
2.7.5 Annexin V/Propidium iodide staining
In order to analyze viability of cells incubated with VZV vaccine or UV-inactivated
CMV, pDCs were stained for Annexin V and Propidium iodide (PI) after 16 or 24 h
of incubation.
Cells were harvested and washed twice with Annexin V binding buffer (Sterofundin
with HEPES buffer 10 mM) (2 ml, 350 g, 7 min, RT) that contained the Ca2+ ions
essential for Annexin V binding to phospholipids on the surface of dying cells.
PBMCs used for compensation with PI, Annexin V and cells for positive controls
were either incubated with 2 µl ethanol (100%) for 20 min or 3 or 5 µl hydrogen
peroxide (H2O2) for 10 min and subsequently adapted to the Annexin V binding
buffer.
Then cells were stained with 1 µl lin 1-FITC and 5 µl Annexin V-APC or 10 µl of a
self-mixed lin 1-APC and 5 µl Annexin V–FITC.
All cells were incubated for 15 min in the dark and samples were washed once,
supernatant was aspirated except for about 200 µl and cells were at once
analyzed via flow cytometry. Shortly before analysis with the flow cytometer,
PI (5 µg/ml) was added to each sample separately.
25
2.8 Data analysis and statistics
Data of the experiments are demonstrated as averages ± standard error of mean
(SEM). For the analysis of statistical differences the paired two-tailed student’s t
test was applied. *p-values < 0.05 were considered as statistically significant. Data
and statistics were analyzed with Microsoft Excel 2007 and 2010 and Microsoft
Powerpoint 2007 and 2010 (both Microsoft Corporation, Redmond, WA, USA).
26
3 Results
3.1 Herpesviruses modulate granzyme B production and secretion by
plasmacytoid dendritic cells in a concentration-dependent manner
In order to test the hypothesis that viral stimuli with a dsDNA genome can
modulate GrB production and secretion by pDCs we first tested a vaccine
containing live attenuated varicella-zoster virus (VZV). The varicella-zoster virus
vaccine Varilrix® was used in different volume dilutions of the reconstituted
lyophilized vaccine. Additionally we analyzed the effects of UV-inactivated human
cytomegalovirus (CMV) on pDCs in different multiplicities of infection (MOIs) and
the effects of Epstein-Barr virus (EBV) in different volume dilutions of the virus
stock.
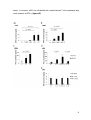
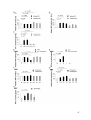
Intracellular GrB production was determined via flow cytometry as MFI of GrB in
the viable, lin 1-, CD123+ cell gate. The gating strategy is shown in figure 1A.
When purity of pDCs was very high and staining for additional surface molecules
required the application of other mAb the MFI of GrB was measured in the viable
cell gate alone.
Freshly isolated pDCs (d0) already produced a small amount of GrB that was not
markedly increased after 16 h of incubation in AIM-V medium alone (figure 1B).
Moreover, pDCs incubated in AIM-V medium alone produced the same amounts
of GrB as pDCs incubated in AIM-V medium with VZV in increasing concentrations
(figure 1B). In contrast, IL-3 incubation led to a markedly higher GrB production
than medium alone. This production could be modulated by VZV in a
concentration-dependent manner. Therefore, all other experiments with pDCs and
VZV, UV-inactivated CMV or EBV were only done with pDCs incubated in IL-3supplemented medium or only data with pDCs incubated in IL-3-supplemented
medium are shown.
Whereas the VZV vaccine dilutions 1:2000 and 1:200 had no marked effect on
GrB production and secretion by pDCs incubated in IL-3-supplemented medium
compared to that of pDCs incubated with IL-3 alone, the vaccine dilutions 1:20 and
1:10 significantly reduced GrB production in pDCs [47]. PDCs treated with VZV in
a volume dilution of 1:10 produced 28.63 ± 3.14% less GrB than pDCs treated with
IL-3-supplemented medium alone. This decrease of GrB production in pDCs
27
incubated with VZV in high concentrations was observed after 24 h of incubation
as well (data not shown).
To verify these results, GrB secretion by pDCs upon viral stimulation was
measured with a GrB-specific ELISA after 16 h of incubation of pDCs. GrB
secretion by pDCs was decreased in a concentration-dependent manner by VZV
vaccine dilutions 1:20 and 1:10 [47]. PDCs incubated with vaccine dilution 1:10
secreted significantly less GrB; GrB secretion was reduced to 76.67 ± 10.57%
(figure 1C). Thus the suppressive effect of VZV on GrB observed via flow
cytometry could be reproduced using a GrB ELISA.
As a second step we looked at the effects of human UV-inactivated
cytomegalovirus (CMV). PDCs incubated with CMV produced less GrB in a dosedependent manner. PDCs incubated with MOI 1 and MOI 10 produced
significantly less GrB than pDCs incubated with IL-3-supplemented medium alone.
MOI 1 reduced GrB production to 76.32 ± 3.89% and MOI 10 to 41.69 ± 2.55%
compared to IL-3-supplemented medium alone (figure 1D). Similar to GrB
production, GrB secretion by pDCs was dose-dependently decreased by UVinactivated CMV. PDCs incubated with CMV in MOI 1 and 10 secreted significantly
less GrB; GrB secretion was diminished to 69.13 ± 5.64% (CMV MOI 1) and to
55.23 ± 6.41% (CMV MOI 10) (figure 1E).
Third, we analyzed another dsDNA virus: Epstein-Barr virus (EBV). In contrast to
VZV and CMV, EBV increased GrB production by pDCs in a concentrationdependent manner. The volume dilution EBV 12.5 increased GrB production
compared to pDCs incubated in IL-3-supplemented medium alone about 12.39 ±
4.73%, pDCs incubated with EBV 25 produced 26.51 ± 7.75% more GrB. EBV 50
did not further enhance GrB production by pDCs (figure 1F). In analogy to GrB
production, GrB secretion was also dose-dependently higher when pDCs were
stimulated with EBV. EBV 50 represented the most potent stimulus and increased
GrB secretion by pDCs to 291.70 ± 50.12% (figure 1G).
28
29
Figure 1: Varicella-zoster virus and cytomegalovirus decrease whereas Epstein-Barr virus
increases plasmacytoid dendritic cell-derived granzyme B.
(A+B) Plasmacytoid dendritic cells (pDCs) were incubated for 16 h in AIM-V medium (M) without
(w/o) interleukin (IL)-3 and with varicella-zoster virus (VZV) vaccine in the indicated volume
dilutions or in IL-3-supplemented AIM-V medium with VZV. Granzyme B (GrB) production was
determined via flow cytometry. (A) Representative dot plots of one donor and gating strategy are
+
shown. PDCs for isotype control (Iso) were treated with IL-3 alone. Dot plots show GrB cells in the
-
+
viable, lin 1 , CD123 gate. (B) Bar graphs show average values of median fluorescence intensity
(MFI) of intracellular GrB relative to pDCs incubated in M with IL-3 from at least three different
donors. The d0 sample was stained directly after positive selection of pDCs. Error bars indicate
standard error of mean (SEM), *p-values indicate significant differences compared to pDCs
incubated in M with IL-3. (C) GrB secretion was measured by enzyme-linked immunosorbent assay
(ELISA) in the supernatant of pDCs incubated for 16 h in IL-3-supplemented medium (M) with VZV
in the indicated dilutions. Bar graphs represent average values relative to M of at least nine
different donors. Error bars indicate SEM, *p-values indicate significant differences compared to M.
(D+E+F+G) PDCs were incubated for 16 h in IL-3-supplemented medium (M) with UV-inactivated
human cytomegalovirus (CMV) in the indicated multiplicities of infection (MOIs) or with Epstein-Barr
virus (EBV) in the indicated volume dilutions. (D+F) GrB production was determined via flow
cytometry after intracellular staining. Bar graphs show average values of MFI of intracellular GrB
relative to M from at least 17 different donors for CMV and from at least nine different donors for
EBV. Error bars indicate SEM, *p-values indicate significant differences compared to M.
(E+G) GrB secretion was measured in supernatant by ELISA. Bar graphs represent average values
relative to M of at least 12 different donors for CMV and of 10 different donors for EBV. Error bars
indicate SEM, *p-values indicate significant differences compared to M. (B+C) See [47].
30
3.2 Human immunodeficiency virus 1 increases granzyme B secretion
by plasmacytoid dendritic cells
In a separate set of experiments we investigated the effects of the ssRNA
retrovirus human immunodeficiency virus 1 (HIV-1) on human pDCs. PDCs were
incubated for 16 h with different cytokines and 5 ng/ml p24 antigen HIV-1. GrB
production was analyzed with flow cytometry after staining for intracellular GrB. As
expected pDCs incubated only in AIMV-medium or with different cytokines
produced different amounts of GrB: PDCs incubated with medium alone or with
IL-10 25 ng/ml for 16 h did not produce more GrB than freshly isolated pDCs.
PDCs incubated with IL-3 or a combination of IL-3 and IL-10 (IL-3 + IL-10)
produced high amounts of GrB at comparable levels. When pDCs were
additionally stimulated with HIV-1 for 16 h, no difference of intracellular GrB to
incubation without HIV-1 could be seen for all incubation conditions (figure 2A).
As another readout we measured GrB secretion in the supernatant of pDCs
incubated with different cytokines and HIV-1. In contrast to flow cytometry data
measuring intracellular GrB production, the ELISA showed differences between
the GrB secretion by pDCs incubated with or without HIV-1. HIV-1 increased GrB
secretion in all the different incubation conditions (figure 2B and 2C) particularly
when HIV-1 was combined with IL-3 or IL-3 + IL-10 [88]. PDCs incubated with IL-3
and HIV-1 secreted 39.65 ± 8.42% more GrB than pDCs incubated with IL-3 alone
and IL-3 + IL-10 plus HIV-1 pDCs secreted 44.15 ± 7.46% more GrB than pDCs
incubated with IL-3 + IL-10 alone (figure 2B).
31
Figure 2: Human immunodeficiency virus 1 increases granzyme B secretion by
plasmacytoid dendritic cells.
Plasmacytoid dendritic cells (pDCs) were incubated for 16 h in medium (M) supplemented with
different cytokines and with or without (w/o) 5 ng/ml human immunodeficiency virus 1 (HIV) as
indicated. (A) Granzyme B (GrB) production was determined via flow cytometry after intracellular
staining. The d0 sample was stained directly after positive selection of pDCs. Bar graphs show
average values of median fluorescence intensity (MFI) of intracellular GrB relative to M with
interleukin (IL)-3 w/o HIV. Averages are derived from at least three different donors. Error bars
indicate standard error of mean (SEM). (B+C) GrB secretion was measured in supernatant by
enzyme-linked immunosorbent assay (ELISA). (B) Bar graphs represent average values relative to
IL-3 w/o HIV. Averages are derived from at least five different donors. Error bars indicate SEM, *pvalues indicate significant differences compared to samples incubated w/o HIV. (C) Bar graphs
represent average values relative to M w/o HIV. Averages are derived from at least five different
donors. Error bars indicate SEM. (B) See [88].
32
3.3 Regulation of granzyme B production and secretion by
plasmacytoid dendritic cells is virus-specific
As the two summary figures show, UV-inactivated CMV had a higher potential
than vaccine-derived VZV to decrease GrB production and secretion by pDCs.
EBV potently induced GrB secretion by pDCs and also to some extent GrB
production. HIV-1 also had the potential to increase GrB secretion but it did not
alter GrB production compared to pDCs incubated with IL-3 alone (figure 3A and
3B).
Figure 3: Virus-induced regulation of granzyme B production and secretion by plasmacytoid
dendritic cells varies between different viruses.
The figures summarize the results from figure 1 and 2. Data are the same as in figure 1 and 2.
Averages are derived from the same experiments. Plasmacytoid dendritic cells (pDCs) were
incubated for 16 h in interleukin (IL)-3-supplemented medium (M) with varicella-zoster virus (VZV)
vaccine or Epstein-Barr virus (EBV) in different volume dilutions, alternatively with UV-inactivated
human cytomegalovirus (CMV) in different multiplicities of infection (MOIs) or human
immunodeficiency virus 1 (HIV). (A) Bar graphs show average values of median fluorescence
intensity (MFI) of intracellular granzyme B (GrB) production relative to M. (B) GrB secretion was
measured in supernatant by enzyme-linked immunosorbent assay (ELISA). (A+B) Bar graphs
represent average values relative to M. Error bars indicate standard error of mean, *p-values
indicate significant differences compared to M.
33
3.4 Interferon-α secretion by plasmacytoid dendritic cells is
differentially regulated by viruses
Next, we tested the potential of the four different viral stimuli to induce IFN-α in
pDCs, the typical type I interferon produced by pDCs upon viral stimulation.
IFN-α secretion was measured with an IFN-α-specific ELISA in the supernatant
after 16 h of incubation of pDCs in the presence or absence of different viruses.
PDCs incubated with IL-3 alone do hardly produce any IFN-α. In contrast, pDCs
incubated for 16 h with VZV in increasing concentrations secreted high amounts of
IFN-α. PDCs incubated with VZV 1:10 secreted 9.71 ± 3.50 ng/ml IFN-α (figure
4A). As the high standard error of mean (SEM) shows, we observed in general a
high variance in IFN-α secretion between the different donors. As a positive
control for IFN-α secretion by pDCs, we used 1 µg/ml CpG A as a potent inductor
of IFN-α production in pDCs. Data are not shown because IFN-α secretion by CpG
A-stimulated pDCs was too high to measure within the area of our standard curve.
UV-inactivated human CMV also induced potent IFN-α secretion in pDCs: PDCs
stimulated with CMV MOI 10 produced 24.29 ± 1.62 ng/ml IFN-α (figure 4B).
EBV induced only little IFN-α secretion by pDCs: EBV 25 induced 71.06 ± 29.89
pg/ml IFN-α and EBV 50 126.46 ± 6.6 pg/ml IFN-α. For EBV these are data from
three different donors (figure 4C). In another experiment with another EBV stock
we could not detect any IFN-α production by pDCs.
In the experiments intended for the analysis of the effect of HIV-1 on IFN-α
secretion by pDCs, pDCs incubated with AIMV-medium alone or only with the
cytokines IL-3, IL-10 or IL-3 + IL-10 did hardly produce any IFN-α. When pDCs
were additionally incubated with HIV-1 IFN-α secretion was slightly increased by
medium-pDCs and by IL-10-pDCs (80.62 ± 23.77 pg/ml), more obviously in the
presence of IL-3 (151.55 ± 34.51 pg/ml) and most with IL-3 + IL-10 (284.35 ±
136.35 pg/ml), even though the IFN-α amounts remained on a low level (figure
4D). We also tested IFN-α secretion in mixed lymphocyte reactions (MLRs) with
pDCs and HIV-1- or mock-infected CD4+ T cells. After 16 h of co-culture pDCs coincubated with HIV-1-infected T cells produced markedly high amounts of IFN-α.
The IFN-α amounts produced in these MLRs were - even in high dilutions - too
high to measure correctly within our applied high sensitivity standard curve. As a
consequence the results for IFN-α in these MLRs can only give hints of the actual
34
values. In contrast, pDCs co-incubated with mock-infected T cells produced only
small amounts of IFN-α (figure 4E).
35
Figure 4: Regulation of interferon-α secretion by plasmacytoid dendritic cells is virusdependent.
(A+B+C+D) Plasmacytoid dendritic cells (pDCs) were incubated for 16 h in interleukin (IL)-3supplemented medium (M) with varicella-zoster virus (VZV) vaccine or Epstein-Barr virus (EBV) in
the indicated volume dilutions, UV-inactivated human cytomegalovirus (CMV) in the indicated
multiplicities of infection (MOIs) or with different cytokines and with or without (w/o) 5 ng/ml human
immunodeficiency virus 1 (HIV) as indicated. Supernatant was harvested after 16 h of incubation
and interferon (IFN)-α concentration in supernatant was measured with an IFN-α-specific enzymelinked immunosorbent assay (ELISA). (A+B) Bar graphs represent average IFN-α concentrations in
ng/ml in supernatants from four different donors for VZV and from at least four different donors for
CMV. (C+D) Bar graphs represent average IFN-α concentrations in pg/ml in supernatant from three
different donors for EBV and from at least five different donors for HIV-1. (A+B+C+D) Error bars
represent standard error of mean. (A+B+C) *p-values indicate significant differences compared to
M. (D) *p-values indicate significant differences compared to samples w/o HIV. (E) PDCs were
either incubated alone with different cytokines or with different cytokines and HIV-1- or mock+
infected CD4
T cells. After 16 h of co-incubation supernatant was harvested and IFN-α
concentrations were measured with an IFN-α-specific ELISA. Bar graphs represent average IFN-α
concentrations in ng/ml in supernatants from at least 6 different pDC:T cell donor combinations.
Error bars represent standard error of mean, *p-values are not shown due to measurement of MLR
values out of range of the ELISA standard curve.
36
3.5 Herpesviruses modulate the expression of distinct surface
molecules on plasmacytoid dendritic cells
To test the expression of different surface molecules on pDCs stimulated with
VZV, CMV or EBV, surface molecule expression was measured via flow cytometry
after 16 h of incubation. Surface molecule expression was assessed most times as
MFI in the viable, lin 1-, CD123+ cell gate but if purity of pDCs was high and
staining for additional surface molecules required the application of other mAbs,
surface molecule expression was determined after gating on viable cells. The
antigen-presenting molecule HLA-ABC was expressed at higher levels on pDCs
incubated with VZV than on pDCs incubated with IL-3 alone [47]. The
concentrations 1:200, 1:20 and 1:10 had the same effect on the surface
expression of HLA-ABC on pDCs. VZV 1:10 increased HLA-ABC expression by
83.41 ± 16.97% (figure 5A). The intercellular adhesion molecule CD54 was also
slightly higher expressed on pDCs incubated with VZV [47] in increasing
concentrations than on pDCs incubated with IL-3 alone: PDCs incubated with VZV
1:20 expressed 46.55 ± 10.69% more CD54, VZV 1:10 had a similar effect and led
to 45.11 ± 9.77% more CD54 expression than on pDCs incubated with IL-3supplemented medium alone.
In contrast, pDCs treated with VZV expressed the same levels of the pDC
maturation marker CD83 compared to pDCs incubated with IL-3-supplemented
medium alone [47]. Additionally, neither the high expression of HLA-DR on pDCs
maturated with IL-3 [47], nor the expression levels of the co-stimulatory molecules
CD40 [47], CD80 and CD86 [47] on pDCs incubated with IL-3 alone were further
modulated by VZV.
We also measured the effect of UV-inactivated human cytomegalovirus in different
MOIs on the expression of different surface markers on pDCs after 16 h of
incubation: The antigen-presenting molecule HLA-ABC was expressed at higher
levels on pDCs incubated with CMV. The MOIs 0.1, 1 and 10 almost had the same
effect on the surface expression of HLA-ABC on pDCs. CMV MOI 10 increased
HLA-ABC expression by 138.13 ± 22.00% (figure 5B). The high expression of
HLA-DR on pDCs maturated with IL-3 was not further modulated by CMV as was
the expression of the co-stimulatory molecules CD80 and CD86 not altered by
CMV compared to the incubation of pDCs with IL-3 alone. In contrast to the results
37
for VZV, the expression of the intercellular adhesion molecule CD54 was not
modulated by CMV.
On the contrary, EBV, the third herpesvirus analyzed, again slightly enhanced the
surface expression of the intercellular adhesion molecule CD54 on pDCs. PDCs
stimulated with EBV 25 expressed 9.82 ± 0.87% more CD54 than pDCs stimulated
with IL-3 alone. But EBV did not modulate the expression of the antigenpresenting molecules HLA-ABC and HLA-DR on pDCs compared to the
expression of these molecules on the surface of pDCs incubated in IL-3supplemented medium alone (figure 5C).
38
39
Figure 5: Herpesviruses modulate the expression of human leukocyte antigen (HLA)-ABC
and Cluster of differentiation (CD)54 on the surface of plasmacytoid dendritic cells.
PDCs were incubated for 16 h in interleukin (IL)-3-supplemented medium (M) with varicella-zoster
virus (VZV) vaccine or Epstein-Barr virus (EBV) in the indicated volume dilutions or UV-inactivated
human cytomegalovirus (CMV) in the indicated multiplicities of infection (MOIs). Surface molecule
expression was measured via flow cytometry. Bar graphs represent average values of median
fluorescence intensity (MFI) of human leukocyte antigen (HLA)-ABC, HLA-DR, Cluster of
differentiation (CD)54, CD80, CD86, CD40 and CD83 relative to M from at least three different
donors for VZV and EBV and from at least four different donors for CMV. Error bars indicate
standard error of mean, *p-values indicate significant differences compared to M. (A) See [47].
40
3.6 Herpesviruses and human immunodeficiency virus 1 in the
concentrations or multiplicities of infection used do not relevantly
alter viability of plasmacytoid dendritic cells
To investigate the inhibitory effect of the VZV vaccine and human UV-inactivated
CMV on GrB production and secretion by pDCs we analyzed the viability of pDCs
incubated with increasing concentrations or MOIs of the vaccine or viruses used.
Viability of pDCs incubated for 16 h with different viral stimuli in comparison to
pDCs incubated with IL-3-supplemented medium alone was analyzed via flow
cytometry. Viability was defined by gating on viable cells in the forward scatter
(FSC)/side scatter (SSC) dot plot. Viability of pDCs incubated with IL-3 alone for
16 h was 71.66 ± 2.88% and 69.12 ± 3.24% for pDCs incubated with VZV 1:10.
Overall this analysis showed no decrease in viability between pDCs incubated with
IL-3 alone and pDCs incubated with VZV in increasing concentrations. The volume
dilution 1:200 did even slightly increase viability of pDCs (figure 6A).
CMV MOI 10 slightly reduced viability of pDCs after 16 h of incubation about 6.38
± 2.59% compared to pDCs incubated with IL-3 alone (figure 6D). CMV MOI 0.1
and CMV MOI 1 did not affect viability of pDCs after 16 h.
EBV 12.5 and 25 did not alter viability of pDCs after 16 h of incubation compared
to pDCs incubated in IL-3-supplemented medium alone. EBV 50 slightly
decreased viability of pDCs compared to IL-3-pDCs about 6.60 ± 2.46% (figure
6G).
Incubation with HIV-1 for 16 h did not relevantly alter viability of pDCs if viability of
pDCs incubated with different cytokines but without HIV-1 and the viability of pDCs
incubated with the respective cytokines with HIV-1 are compared. HIV-1 only
slightly enhanced viability of medium-pDCs. As expected, pDCs incubated with
medium alone or only with IL-10 were in general less viable than pDCs incubated
with IL-3 or IL-3 + IL-10 as IL-3 is an important survival factor for pDCs. Compared
to IL-3-pDCs, pDCs incubated with IL-3 + IL-10 with HIV-1 were slightly more
viable (figure 6H).
To confirm that pDCs producing less GrB upon incubation with VZV or CMV were
not just less viable, we performed Annexin V/Propidium iodide (PI) staining. PDCs
were incubated for 16 or 24 h and as for analysis of GrB production, pDCs were
additionally incubated for 4 h with Brefeldin A directly prior to staining for flow
41
cytometry. We first gated on viable, lin 1- cells, then we defined Annexin V-, PIcells in this gate.
PDCs incubated for 16 or 24 h with VZV vaccine in increasing concentrations
showed no relevant decrease in viability (figure 6B and 6C).
Incubation of pDCs with UV-inactivated CMV in different MOIs for 16 or 24 h did
also not affect viability of pDCs compared to pDCs incubated in IL-3-supplemented
medium alone (figure 6E and 6F).
Taken together, the viruses tested have had no significant influence on pDC
survival.
42
43
Figure 6: Herpesviruses and human immunodeficiency virus 1 do not relevantly alter
viability of plasmacytoid dendritic cells in vitro.
(A+D+G) Plasmacytoid dendritic cells (pDCs) were incubated for 16 h in interleukin (IL)-3supplemented medium (M) with varicella-zoster virus (VZV) vaccine or Epstein-Barr virus (EBV) in
the indicated volume dilutions or with UV-inactivated human cytomegalovirus (CMV) in the
indicated multiplicities of infection (MOIs). (H) PDCs were incubated for 16 h in medium alone (M)
supplemented with different cytokines and with or without (w/o) 5 ng/ml human immunodeficiency
virus 1 (HIV) as indicated. (A+D+G+H) PDCs were subsequently stained for intracellular granzyme
B (GrB) and analyzed via flow cytometry. Viable cells were gated in forward/side scatter dot plot.
(A+D+G) Bar graphs represent average values of viable cells relative to M from at least six
independent donors for VZV, from at least 17 independent donors for CMV and from 10 different
donors for EBV. Error bars indicate standard error of mean (SEM), *p-values indicate significant
differences compared to M. (H) Bar graphs represent average values of viable cells relative to IL-3
w/o HIV-1. Averages are derived from at least four different donors for HIV-1. Error bars indicate
SEM, *p-values indicate significant differences compared to the indicated samples incubated
without virus. (B+C+E+F) PDCs were incubated in IL-3-supplemented medium (M) with VZV
vaccine in the dilutions indicated or with UV-inactivated CMV in the MOIs indicated. After 16 or 24
h, pDCs were incubated as for intracellular staining. Subsequently cells were stained with Annexin
V and Propidium iodide (PI) and analyzed via flow cytometry. PDCs were gated as for analysis of
-
-
-
GrB production (viable, lineage cocktail 1 (lin 1) cells) and the percentage of Annexin V , PI cells
was determined. (B+E) Dot plots from one representative donor are shown. Annexin V/PI staining
is shown for M sample and pDCs incubated with VZV 1:10 or CMV MOI 10 for 16 and 24 h. (C+F)
-
-
Bar graphs represent average values of Annexin V , PI cells from at least two donors for VZV. For
CMV averages are derived from three different donors. Error bars indicate SEM.
44
3.7 The viral components of the different herpesvirus sources are
responsible for the effects on plasmacytoid dendritic cells
In order to test whether the viral particles alone and no other ingredients of the
viral agents were responsible for the effects on pDCs we performed studies with
filtrated viral vaccine or virus or culture supernatants of herpesviruses. In the case
of VZV the whole vaccine was filtrated through a virus filter that allowed the
removal of virions. Consequently, the filtrated VZV vaccine contained no more
varicella-zoster virus virions. In contrast to the whole vaccine, the filtrated vaccine
in the highest concentrations used, had no effect on GrB production (figure 7A).
Additionally, the filtrated vaccine did no longer induce HLA-ABC expression on
pDCs (figure 7B). PDCs incubated with the filtrated vaccine still produced some
IFN-α but by far not the same amount as pDCs incubated with the whole vaccine
(figure 7C). However, UV-irradiated non-filtrated VZV vaccine still decreased GrB
production by pDCs and enhanced HLA-ABC expression on pDCs to a similar
extent as the whole vaccine (data not shown). Therefore VZV particles
themselves, not other vaccine components are primarily responsible for pDC
activity modulation.
As CMV was harvested in the supernatant of CMV-infected human foreskin
fibroblasts (HFFs) we used supernatant of non-CMV-infected HFFs in respective
amounts to test the specificity of the negative effect of UV-inactivated CMV on GrB
production by pDCs. PDCs were incubated for 16 h with UV-inactivated CMV in
increasing MOIs or supernatant of HFFs in the respective volume. GrB production
was analyzed via flow cytometry after intracellular staining for GrB. Supernatant of
HFFs did only mildly influence GrB production compared to medium; it did not
decrease GrB production by pDCs but slightly induced GrB production by pDCs:
Surprisingly, HFF supernatants increased GrB production up to 17.83 ± 4.51%
(figure 7D). Hence CMV particles and not supernatant components were needed
for the GrB suppressing effect. To compare the ability of UV-inactivated human
CMV and HFF supernatant to induce IFN-α secretion by pDCs, pDCs were
incubated for 16 h in IL-3-supplemented AIMV-medium with CMV in the indicated
MOIs or with the same amounts of HFF supernatant as a control. The HFF
supernatant control did not induce IFN-α secretion in pDCs (figure 7E).
45
Similar experiments were also performed for EBV. EBV stock was filtered through
a virus filter to remove virions. Filtration abrogated the effect of the EBV stock on
GrB production and secretion and no difference to pDCs incubated in IL-3supplemented medium alone was observed (figure 7F and 7G). As far as IFN-α
secretion is concerned, EBV particles were only weakly capable of inducing IFN-α
in pDCs. In this low range, filtrated EBV supernatant had a similar effect. EBV 50
and filtrated EBV 25 and EBV 25 and filtrated EBV 50 produced the same
amounts of IFN-α in this experiment. These results that detect IFN-α at pg levels
are difficult to compare with the IFN-α-data derived with other viruses that lie in the
ng range. It has also to be pointed out that data are derived from only three donors
and one experiment (figure 7H).
In summary, the virus particles themselves but not the soluble components of the
viral agents were responsible for the effects observed.
46
47
Figure 7: The viral components of the different herpesvirus sources are responsible for the
effects on plasmacytoid dendritic cells.
Plasmacytoid dendritic cells (pDCs) were incubated for 16 h in interleukin (IL)-3-supplemented
medium (M) with the whole varicella-zoster virus (VZV) vaccine or with the filtrated varicella-zoster
virus vaccine in the dilutions indicated, with UV-inactivated human cytomegalovirus (CMV) in the
multiplicities of infection (MOIs) indicated or with supernatant from mock-infected human foreskin
fibroblasts (HFFs) in respective amounts as a control or with Epstein-Barr virus (EBV) or the
filtrated EBV stock in the dilutions indicated. (A+D+F) Granzyme B (GrB) production was
determined via flow cytometry after intracellular staining for GrB. Bar graphs represent average
median fluorescence intensity (MFI) relative to M from at least five different donors for VZV, four
different donors for CMV and from three different donors for EBV. Error bars indicate standard error
of mean (SEM), *p-values indicate significant differences to M. (B) Surface expression of human
leukocyte (HLA)-ABC was determined via flow cytometry. Bar graphs represent average MFI
relative to M from at least four donors. Error bars indicate SEM, *p-values indicate significant
differences compared to M. (C+E+H) Interferon (IFN)-α concentration in supernatant was
measured with an IFN-α-specific enzyme-linked immunosorbent assay (ELISA). (C+E) Bar graphs
represent average IFN-α concentrations in ng/ml in supernatants from four different donors for VZV
and from four different donors for CMV. (H) Bar graphs represent average IFN-α concentrations in
pg/ml in supernatants from three different donors for EBV. (C+E+H) Error bars represent SEM, *pvalues indicate significant differences compared to M. (G) GrB secretion was measured by ELISA
in supernatant of pDCs incubated for 16 h with EBV or the filtrated EBV stock in the dilutions
indicated. Bar graphs represent average values relative to M of three different donors. Error bars
indicate SEM, *p-values indicate significant differences compared to M.
48
3.8 Herpesvirus- or human immunodeficiency virus 1-stimulated
plasmacytoid dendritic cells modulate CD4+ T cell proliferation in
mixed lymphocyte reactions
To analyze the immunostimulatory potential of pDCs primed with different viral
stimuli on CD4+ T cells we performed mixed lymphocyte reactions (MLRs) of CD4+
T cells with allogeneic pDCs pre-incubated with different viruses in high
concentrations for 24 or 48 h. Proliferated CD4+ T cells were defined as CFSElow
cells in viable, CD3+, 7-AAD- gate after six (experiments with VZV and CMV) or
seven days (experiments with EBV and HIV-1) of co-incubation. As a control for
high T cell proliferation CD4+ T cells were stimulated with CD3/28 beads.
Representative histograms of one donor combination show gating strategy and
controls (figure 8A). Representative histograms of one donor combination are
shown for VZV and CMV (figure 8B), EBV (figure 8C) and HIV-1 (figure 8D).
CpG B-activated pDCs were used as a positive control for the potential of
allogeneic pDCs to induce T cell proliferation. CpG B-pre-treated pDCs potently
enhanced CD4+ T cell proliferation in all pDC:T cell ratios.
In the pDC:T cell ratio 1:50 pDCs pre-incubated with VZV 1:20 had a significant
stimulatory effect on T cell proliferation (43.3 ± 4.12%) that was as high as the
effect of CpG B-pre-treated pDCs (44.66 ± 2.94%). VZV 1:10-activated pDCs were
also able to significantly enhance T cell proliferation compared to IL-3-pDCs in the
pDC:T cell ratio 1:50 (VZV 1:10: 30.83 ± 2.60%, IL-3: 20.75 ± 3.16%). In the ratio
1:250 pDCs activated with VZV 1:20 still significantly enhanced T cell proliferation
(27.53 ± 3.87%) compared to IL-3-stimulated pDCs (14.48 ± 3.15%) whereas VZV
1:10 only showed a small additional effect to IL-3 alone with 21.64 ± 2.25% T cell
proliferation [47]. In the pDC:T cell ratio of 1:1250 no difference in the proliferation
of T cells that had been co-incubated with IL-3-stimulated pDCs to the proliferation
of T cells that had been co-incubated with VZV–stimulated pDCs was observed
(figure 8E).
Next we investigated the effect of pDCs pre-treated with UV-inactivated CMV.
PDCs pre-treated with CMV MOI 1 increased T cell proliferation in the pDC:T cell
ratio 1:50 to a higher extent (27.25 ± 3.71% proliferation) than pDCs pre-treated
with IL-3 alone (11.86 ± 2.00%); whereas the immunostimulatory potential of CMVpre-treated pDCs was not as high as the potential of CpG B-pre-treated pDCs
49
(41.85 ± 3.00%). In the pDC:T cell ratio 1:250 a slight positive effect on T cell
proliferation by CMV-pDCs compared to IL-3-pDCs was still found (CMV: 15.58 ±
2.50%, IL-3: 10.66 ± 0.59%). But in the ratio of 1:1250 no difference to IL-3-pretreated pDCs was observed anymore (figure 8F).
In a third step the effect of pDCs pre-treated with EBV on T cell proliferation was
tested. In contrast to pDCs pre-treated with VZV or CMV, pDCs pre-incubated with
EBV did not alter the level of T cell proliferation compared to pDCs incubated in IL3-supplemented medium alone. This result was observed for all pDC:T cell ratios
(figure 8G). PDCs pre-incubated with CpG B, as expected, potently induced T cell
proliferation in all pDC:T cell ratios. Importantly, when pDCs were pre-incubated
with CpG B plus EBV, EBV did reduce the proliferation-inducing potential of CpG
B-pDCs to a small extent (data not shown). Therefore, EBV-pDCs not only fail to
induce T cell proliferation, there is even a trend towards suppression of CpG BpDC-induced T cell proliferation. In order to investigate the inhibitory mechanism
of EBV-pDCs we analyzed GrB production in pDCs used for this MLR after 48 h of
pre-incubation. Concordantly to our previous results, EBV enhanced GrB
production in pDCs and CpG B diminished GrB production in pDCs in the
presence of IL-3. But when pDCs were incubated in the presence of IL-3 plus CpG
B the addition of EBV slightly elevated GrB production again and was able to
counteract the suppressive effect of CpG B on pDC-derived GrB production to a
certain extent (data not shown). Therefore GrB seems to mediate at least in part
the suppressive effect of EBV-pDCs on T cell proliferation.
Finally we analyzed the effect of HIV-1-pre-treated pDCs on T cell proliferation.
The immunostimulatory capacity of pDCs primed for 48 h with 5 ng/ml HIV-1 or
CpG B in the presence of IL-3 was tested in a MLR with allogeneic CD4+ T cells.
PDCs pre-incubated with HIV-1 did not relevantly alter the level of T cell
proliferation compared to pDCs incubated in IL-3-supplemented medium alone,
but as expected T cell proliferation was higher when pDCs were pre-incubated
with IL-3 plus CpG B (figure 8H). When pDCs were pre-incubated in the presence
of IL-3 with CpG B in combination with HIV-1, HIV-1 could not influence the CpG B
effect on pDCs as T cell proliferation remained on a similar level as with CpG BpDCs alone (data not shown). We also analyzed GrB production in pDCs used for
this MLR after 48 h of pre-incubation with different stimuli. GrB production of pDCs
additionally incubated with HIV-1 was similar to those incubated with IL-3 alone.
50
PDCs incubated with CpG B or CpG B + HIV-1 in the presence of IL-3 produced
both markedly less but comparable amounts of GrB (data not shown).
In summary, VZV- and CMV-pDCs markedly induced CD4+ T cell proliferation,
whereas EBV- and HIV-1-pDCs were not capable of provoking such a T cell
response. The inhibitory effect of EBV- and HIV-1-pre-incubated pDCs seems to
be mediated, at least to some extent, by GrB.
51
52
Figure 8: Herpesvirus- or human immunodeficiency virus 1-stimulated plasmacytoid
+
dendritic cells modulate CD4 T cell proliferation.
Plasmacytoid dendritic cells (pDCs) were pre-incubated for 24 or 48 h in interleukin (IL)-3supplemented AIM-V medium (M) with a class B cytosine-phosphate-guanine oligonucleotide (CpG
B) (2.5 µg/ml), varicella-zoster virus (VZV) vaccine in the concentrations indicated, UV-inactivated
human cytomegalovirus (CMV) in the multiplicity of infection (MOI) 1, Epstein-Barr virus (EBV) in
the indicated volume dilutions or with 5 ng/ml human immunodeficiency virus 1 (HIV). PDCs were
washed and co-incubated with isolated carboxyfluorescein diacetate succinimidyl ester (CFSE)+
+
stained Cluster of differentiation (CD)4 T cells in a pDCs to CD4 T cell ratio of 1:50, 1:250, 1:1250
for six days (VZV-pDCs and CMV-pDCs) or for seven days (EBV-pDCs and HIV-1-pDCs). As
+
controls CD4 T cells were incubated alone or with CD3/28 beads. In order to identify viable 7-
+
amino-actinomycin D (7-AAD) , CD3 T cells, co-cultures were harvested and either stained for
CD3 and 7-AAD or gating on viable cells in the forward/side scatter dot plot was performed. T cell
low
proliferation was measured via flow cytometry. Proliferating T cells were determined as CFSE
cells. (A) Representative dot plots and histograms for gating strategy and for controls are shown.
(B+C+D) For each virus representative histograms of one experiment are shown. Data represent
low
viable CFSE
+
low
CD4 T cells. (E+F) Bar graphs represent average values of CFSE
+
CD4 T cells
after co-incubation with VZV-pDCs or CMV-pDCs from at least four donor combinations of at least
low
one independent experiment. (G+H) Bar graphs represent average values of CFSE
+
CD4 T cells
after co-incubation with EBV-pDCs or HIV-1-pDCs from one independent experiment each.
(E+F+G+H) Error bars indicate standard error of mean, *p-values indicate significant differences to
+
CD4 T cells co-cultured with pDCs pre-incubated in M. Some error bars are not shown due to the
fact that some bar graphs represent average values from less than 3 donors. (E) See [47].
53
4 Discussion
4.1 Virus-specific regulation of granzyme B in plasmacytoid dendritic
cells
Plasmacytoid dendritic cells are essential for linking innate and adaptive immunity
in viral infections. Our group recently described pDCs as potent sources of GrB
[87] and that anti-viral vaccines against ssRNA viruses modulate GrB production
by pDCs [47]. In the present study we investigated the effects of different dsDNA
herpesviruses, VZV, CMV and EBV, and the (+) ssRNA retrovirus HIV-1 on pDCs.
Especially the potential of these four viruses to regulate GrB production and
secretion by pDCs was analyzed.
PDCs were routinely incubated with IL-3 as IL-3 is a potent survival factor for
pDCs in cell culture [64]. IL-3 induces substantial GrB production in pDCs. But, as
has previously been shown, the combination of IL-3 and IL-10 has proven to be
the most potent stimulus to induce GrB production in pDCs [87].
The α-herpesvirus VZV in form of a live attenuated virus strain derived from the
commercially available vaccine Varilrix® reduced GrB production and secretion by
pDCs [47]. UV-inactivated human β-herpesvirus cytomegalovirus (CMV), named in
this section UV-CMV, reduced GrB production and secretion by pDCs as well. In
contrast, the γ-herpesvirus EBV did enhance GrB production and notably GrB
secretion by pDCs. The mechanisms why herpesviruses can have a contrasting
impact on pDC-derived GrB are not understood. One could speculate that IL-10
plays a role in this system. IL-10 was previously shown as a potent stimulus for
GrB production in pDCs when added to IL-3 [87]. It was observed that EBV
produces a homologue of human IL-10 encoded by the gene BCRFI [174], a late
transcript during viral replication [82]. It may be possible that the EBV stock used
for our study contained a viral (v) IL-10. This vIL-10 might be an additional factor
influencing the stimulatory potential of EBV on GrB expression in pDCs. But CMV
can encode a homologue of human IL-10, cmvIL-10, as well [95]. We did not rule
out that cmvIL-10 might be a component of the CMV virus stock we used and
might have influenced our results. Nonetheless CMV inhibited GrB production by
pDCs in contrast to our previous results derived from IL-10-stimulated pDCs. In
consequence, it will be important for future experiments to measure IL-10 in viral
54
stocks to determine the role of IL-10 in the regulation of GrB production and
secretion by different herpesviruses.
In a separate set of experiments, we analyzed the regulation of pDC-derived GrB
by the (+) ssRNA retrovirus HIV-1. Our data demonstrate that HIV-1 induced a
higher GrB secretion in pDCs [88] but did not alter the GrB production levels in
pDCs. The difference in the results obtained by intracellular staining for GrB
versus measuring of GrB in the cell-free supernatant by ELISA might be due to the
different readouts in the two methods: ELISAs measure the extracellular
accumulation, intracellular staining detects intracellular accumulation of GrB in
pDCs only in a very short phase. For intracellular staining it is therefore more
difficult to determine the optimal time point to detect possible differences in GrB
production. Hence, we conclude it may be more reliable and comparable to
measure GrB secretion via ELISA. Another possibility could be that HIV-1 triggers
higher secretion of GrB by pDCs. Despite potential higher production of GrB in
pDCs after stimulation with HIV-1 compared to stimulation with different cytokines
alone, equal levels of GrB are measured inside the cells of the two culture
conditions, because more GrB leaves the cell in the HIV-1-treated pDC-cultures. In
favor of our result that HIV-1 increases GrB secretion by pDCs one group
measured slightly increased GrB levels in the plasma of asymptomatic HIVinfected individuals (n= 25). The median GrB level was 20 pg/ml and ranged from
1-52 pg/ml. The median GrB level in plasma of 54 healthy control subjects was
11.5 pg/ml [161].
Other authors were not able to detect elevated GrB levels in HIV infection. In their
study, GrB levels in plasma of HIV+ patients receiving highly active anti-retroviral
therapy (HAART) or not were at the detection limit of the ELISA with 8.78 pg/ml
GrB [142].
In conclusion, our results show that GrB production and secretion is virusspecifically regulated in pDCs. The mechanisms underlying this differential
regulation are not known to date. Additional virus-specific factors like for example
IL-10 might play a regulatory role. The detection of differences in GrB production
and secretion by pDCs upon viral stimulation seems to be readout- and timepointdependent and the assessement of GrB levels in vivo in infected individuals would
be interesting for the future.
55
To rule out possible cytotoxic effects of the viral stimuli on pDCs, viability assays
were performed. The four viruses examined did not have relevant effects on pDC
viability. These results are in line with recent findings of our workgroup that antiviral vaccines containing ssRNA viruses do not relevantly alter viability of pDCs
[135]. The viral stimulus VZV was derived from a commercially available vaccine.
But as expected the vaccine compounds other than viral particles had no effect on
pDC GrB production and secretion, pDC phenotype and IFN-α secretion as no
known vaccine adjuvant is included in the VZV vaccine formulation. In addition,
our workgroup could recently show that even the common potent vaccine adjuvant
aluminium hydroxide does not relevantly affect GrB production in IL-3-pDCs and
does not induce IFN-α production in pDCs [47].
4.2 Interaction of viruses with plasmacytoid dendritic cells
In order to further decipher the virus-specific regulation of GrB in pDCs, it is
important to shed light on the interaction of the four viruses tested with pDCs and
the mechanisms of recognition of the different viral agents by pDCs. First, it is
important to analyze whether pDCs are permissive for the viruses tested, in other
words, whether the viruses tested can replicate in pDCs and can thereby
circumvent the anti-viral host defence.
Concerning the interaction of VZV and pDCs, one study analyzed DCs in biopsies
of varicella or herpes zoster lesions [81]. The VZV immediate-early (IE) antigens
IE62 and ORF4 were detected in some infiltrating pDCs as a hint for productive
infection of pDCs by VZV. In vitro experiments supported this observation as a
relevant percentage of isolated pDCs were permissive for productive infection by
VZV derived from VZV-infected HFFs: After co-culture of pDCs with VZV-infected
HFFs early and late VZV antigens were detected in pDCs in vitro [81]. Therefore
active VZV can infect pDCs. But data for the live-attenuated varicella-zoster virus
vaccine that show productive infection of pDCs do not exist.
Active CMV was shown to establish a non-permissive infection in peripheral blood
pDCs as only IE-antigens were expressed in pDCs [171]. Another study compared
two subsets of pDCs, peripheral blood pDCs and tonsillar pDCs [154]. PDCs from
peripheral blood were again non-permissive for CMV with the expression of an IE
protein only, whereas surprisingly tonsillar pDCs were permissive since an IE and
56
one late protein were detected. Nonetheless, infection rates were low at 1-10% for
tonsillar pDCs [154]. Consequently, we suppose that CMV and especially UV-CMV
are not able to productively infect peripheral blood pDCs.
EBV was shown to enter pDCs upon interaction of its gp42 protein [144] with HLADR on pDCs [144, 155]. Whether EBV is afterwards able to establish a productive
infection in pDCs is to date not clear as two authors did not observe infection of
pDCs by EBV [50, 144] whereas others report that even latency genes were
detected in pDCs indicating productive infection of pDCs by EBV [155]. For our
experimental setting it is important to note that EBV can specifically enter pDCs.
The mechanism by which HIV usually interacts with susceptible cells is well
described: HIV first attaches to a CD4 receptor on the target cell and then to one
of the co-receptors of CD4, either to the C-C chemokine receptor (CCR) type 5
(R5-tropic HIV strains) or to the C-X-C chemokine receptor (CXCR) type 4 (R4tropic HIV strains) [56]. PDCs are per se susceptible for HIV as they express CD4
and the co-receptors CCR5 and CXCR4 on their surface [140]. HIV enters pDCs
via endocytosis upon interaction of gp120 with CD4 [10]. Some authors describe a
productive infection of pDCs by HIV-1 [54, 139], others deny it [33]. One study
found that HIV-1-infected thymic pDCs transmitted a R5-tropic HIV strain to
thymocytes and blood pDCs transmitted a R5-tropic HIV strain to PBMCs [46].
Furthermore, blood pDCs were shown to transfer both R5- as well as X4-tropic
strains to CD4+ T cells after being infected themselves [114]. In conclusion, the
endocytosis of HIV-1 by pDCs is well described in the literature but whether pDCs
are permissive for HIV-1 infection remains elusive to date. Some data point
towards productive infection of pDCs as HIV can be transmitted to other immune
cells by pDCs.
To summarize all the aspects, it is not certain whether the live attenuated VZV
strain was able to replicate in pDCs in our setting. But certainly, the vaccine strain
had measurable effects on pDCs. Peripheral blood pDCs should not be infected by
CMV, and especially not by UV-CMV. Again we could detect relevant impact on
pDCs by UV-inactivated CMV. The literature about infection of pDCs by EBV is not
unambiguous but it is certain that EBV can enter pDCs and is thereby able to
influence pDCs. Concerning HIV-1, one has to presume that pDCs get infected by
this retrovirus and the expression of the relevant receptors on the surface of pDCs
ensures the entry of HIV particles into pDCs. We hypothesize that permissiveness
57
of pDCs towards the different viruses additionally influences the modulation
pattern of the viral agents on pDCs.
Next, the potential of recognition of attenuated or UV-inactivated viruses by pDCs
will be discussed. As pDCs express both TLR7 and TLR9 [89, 92], they are per se
well equipped to recognize ssRNA and dsDNA viruses. As the source of VZV, we
applied a live attenuated virus strain of a vaccine while the human CMV strain was
UV-inactivated (in this section named UV-CMV). Literature provides evidence that
viral recognition by pDCs through TLR7 and TLR9 does not require active
replication of viruses in pDCs:
One model shows that pDCs internalize virions into the endosomal compartment
where these virions are proteolytically degraded to unmask viral RNA or DNA for
recognition by endosomal TLRs [35]. In this way, viruses might be recognized
without replication in pDCs even in the case of non-permissiveness of pDCs for
these viruses [141]. Additionally per se replication-defective viruses, such as UVinactivated or live attenuated viruses might be recognized. A study in mice showed
that recognition of viruses by TLR7 was independent of the replication of the virus.
In addition, TLR signaling and IFN feedback signaling prohibited viral replication. If
one of these pathways was defective, virus replicated and induced IFN production
in pDCs signaling via retinoic acid-inducible gene 1 (RIG-I)-like helicase (RLH)
receptors [97]. Overall, according to one review [164], RLHs do not play an
essential role for the activation of pDCs. The results of an additional study show
that autophagy might be another possible way of delivery of viral antigens to
endosomal TLRs. Vesicular stomatitis Indiana virus (VSV) RNA replication
intermediates from the cytosol of pDCs were brought to endosomes via fusion with
autophagosomes [102]. Using this pathway, endosomal TLRs might not only
detect pathogens from the extracellular but also from the intracellular
compartment, hypothesize other authors [141]. Normally, viral recognition by pDCs
happens replication-independent but some viruses might be detected replicationdependent with the help of autophagy and TLR signaling or via cytosolic RLH
signaling [97]. In consequence, the live attenuated varicella-zoster virus strain and
UV-CMV should be detected by pDCs in a replication-independent pathway.
Third, the known signaling pathways of the viruses used in this study in pDCs are
of great interest. One study with PBMCs also used Varilrix® in a volume dilution of
1:20 as a source of VZV [179]. IFN-α secretion by PBMCs was partly dependent
58
on TLR9 signaling. As UV-inactivated VZV induced less IFN-α in PBMCs, the
authors concluded that other pathways than signaling via TLR9 might contribute to
the recognition of VZV replication and subsequent induction of IFN-α. They found
that protein kinase R (PKR), known to recognize dsRNA, was partly responsible
for IFN-α secretion by PBMCs upon stimulation with VZV as the inhibitor
2-aminopurine potently abrogated IFN-α secretion in VZV-stimulated PBMCs
[179]. But as all experiments in this study were conducted with PBMCs, the exact
pathway responsible for IFN-α production by pDCs upon interaction with VZV
cannot be concluded from the results [179].
Another study investigated the signaling pathway of human CMV in pDCs [171].
CMV induced IFN-α in pDCs via TLR9 and/or TLR7. Which endosomal TLR
receptor was responsible for signaling is not clear as an antagonist for both
endosomal TLR receptors was used in this study to investigate the mechanistics
behind IFN-α induction in pDCs by CMV [171].
Data concering EBV showed that EBV is able to signal through TLR9 in a
replication-independent manner to induce IFN-α secretion by pDCs [50, 144] but
TLR7 seemed to contribute to IFN-α secretion by pDCs upon EBV-stimulation as
well [50, 155]. During latency in B cells, small RNAs, the so called EBERs
containing ssRNA motifs, are synthesized [107]. These molecules alone were also
able to stimulate pDCs to secrete IFN-α via TLR7 and are discussed to be also
responsible for signaling of EBV in pDCs via TLR7 [107]. Other authors suppose
as well that autophagy plays a role in the detection of EBV by pDCs. Both theories
imply that replication of EBV in the cytosol is required for recognition of the virus
by pDCs [155].
The recognition of HIV-1 by pDCs is mediated by the interaction of HIV RNA with
TLR7 after receptor-mediated endocytosis of the virion [10]. Interestingly, pDCs
are also able to sense HIV from HIV-infected cells. Lymphocytes infected with HIV,
even with replication-deficient virus, seem to be detected through TLR7 [106]. The
exact mechanism is to date unknown but depends on cell contact and endocytosis
processes [153].
In conclusion, literature shows that pDCs are able to recognize VZV, CMV, EBV
and HIV-1 through endosomal TLR pathways. According to the results of several
authors, even replication-defective viruses such as the vaccine-derived VZV and
UV-CMV should be sensed by TLRs in pDCs. Therefore we assume that some of
59
our results might be TLR-dependent. In future experiments the influence of
specific antagonists for TLRs on the viral effects on pDCs should be analyzed.
4.3 Modulation of plasmacytoid dendritic cell interferon-α production
and phenotype by viruses
As pDCs are known to be the major producers of type I IFNs in viral infections
[159] we analyzed the effect of viral stimuli on IFN-α secretion by pDCs. The live
attenuated VZV strain did induce potent IFN-α secretion by pDCs. This is in line
with a study which tested the effect of Varilrix® on PBMCs and identified pDCs as
the main producers of IFN-α upon VZV stimulation. UV-inactivated VZV was
markedly less efficient in inducing IFN-α production in PBMCs [179]. In contrast to
these and our observations, other authors found that pDCs co-cultured with VZVinfected HFFs secreted hardly any IFN-α [81]. Even a TLR9 agonist could not
overcome the IFN-α secretion-inhibiting VZV stimulus when added to the coculture. This observation was not due to impaired viability as Annexin V/PI staining
could not detect different viability of pDCs co-cultured with VZV- or mock-infected
HFFs [81]. When supernatant of co-cultures of pDCs with VZV-infected HFFs was
added to naïve pDCs, these pDCs produced about 5700 pg/ml IFN-α, about half of
the levels we measured. When the TLR9 agonist was added to pDCs stimulated
with co-culture supernatant, the IFN-α amount was approximately as high as when
naïve pDCs were cultured with the TLR9 agonist alone. Consequently the
supernatant of infected pDCs alone could not block TLR9 agonist-induced IFN-α
production by pDCs. Thus, the authors hypothesize that VZV-infected pDCs
suppressed IFN-α production in uninfected bystander pDCs in a cell-contact
dependent manner [81]. The differing results for IFN-α secretion upon VZV
stimulation in this study might be a consequence of the use of cell-based HFFderived VZV. The authors assume that HFF-derived VZV might express
proprietary regulatory proteins upon productive infection of pDCs that could
influence the IFN response [81]. In contrast, the vaccine strain we used is
attenuated and should not establish productive infection in pDCs, another factor
that might explain the differing results. Unfortunately the authors did not check for
IFN-α or free VZV particles in the supernatant of VZV-infected HFFs that might be
responsible for the induction of IFN-α production in pDCs in the supernatant
60
experiments. A role of VZV-infected HFFs for inhibition of IFN-α production in cocultured pDCs is also possible. Overall, we assume that an experimental setting
with cell-free virus to rule out side effects of feeder cells is more reliable to assess
the direct effect of viruses on pDCs.
In our hands, UV-inactivated human CMV markedly increased IFN-α in a dosedependent manner and it was the most potent stimulus of all four viruses tested for
pDC-IFN-α secretion. PDCs also produced high amounts of IFN-α in response to
active CMV in another study [99]. Interestingly, others showed that IFN-α
production was on a similar level whether pDCs were stimulated with CMV or UVinactivated CMV. Moreover pDCs stimulated with CMV or UV-inactivated CMV
secreted comparable amounts of IL-6, TNF-α, IL-10 and CCL3 [171].
In our experiments, EBV was only able to induce minimal amounts of IFN-α.
Similarly, one paper showed that EBV induces some but not potential IFN-α
secretion by pDCs [144]. In addition, one group found that EBV-stimulated pDCs
were also able to secrete IL-6 and IL-8 in a TLR9-dependent manner [50]. Both
studies found that it made no difference concerning IFN-α amounts whether pDCs
were stimulated with active or UV-inactivated EBV [50, 144]. In contrast to the
relatively low amounts of IFN-α detected by these two groups and us, another
group found that active EBV induced high amounts of IFN-α secretion by pDCs.
Contrary to this, UV-inactivated EBV induced much less IFN-α in their hands [155].
Unfortunately, the amounts of IFN-α presented in the figures of the different
papers are not directly comparable as detailed information about the experimental
settings is not provided. In one study using a comparable experimental set up,
EBV-stimulated pDCs secreted as in our hands only IFN-α in pg levels [108]. Of
note, in this publication, the EBV stock itself contained IL-10 and pDCs stimulated
with EBV produced IL-10 additionally to IFN-α [108]. When an anti-IL-10 mAb was
added to the culture, IFN-α levels increased to approximately 300 pg/ml giving rise
to the suspicion that IL-10 was responsible here for lower IFN-α production by
pDCs. As mentioned above, we did not check for IL-10 in the EBV stock or in the
supernatant of pDCs incubated with EBV. This would be quite interesting to
examine as our EBV stock was produced similarly to the method reported in this
study and IL-10 might also play a role in our experimental setting [108].
In our work, HIV-1 5 ng/ml p24 antigen was a slightly better stimulus than EBV to
induce IFN-α secretion by pDCs, but was still not able to induce potent IFN-α
61
responses by pDCs. But pDCs co-incubated for 16 h with HIV-1-infected CD4+ T
cells did produce relevant amounts of IFN-α although the exact amounts of IFN-α
production in these MLRs remain elusive, as the high IFN-α values measured
were out of range in our ELISA standard curve. Recently it was shown that HIV-1
might delay IFN-α production in pDCs [110]. PDCs that were maturated over night
with IL-3 and consequently stimulated for 4 or 8 h with HIV-1 secreted very small
amounts of IFN-α. The maximum of IFN-α production took place 24 to 48 h after
stimulation with HIV-1. The peak of IFN-α production for other viruses such as
influenza virus was however after 4 h. Overall, even the peak IFN-α secretion by
pDCs challenged with HIV-1 was markedly lower than upon stimulation with the
other viruses tested, such as influenza or Sendai virus [110]. This goes along with
our observation that pDCs stimulated with HIV-1 for 16 h do not produce
substantial IFN-α. One further explanation for these results might be that binding
of HIV gp120 to pDCs could inhibit TLR9 signaling [118]. HIV gp120 was actually
able to bind to BDCA-2 on pDCs [118] ligation of which is known to inhibit IFN
production by pDCs [45].
In another study, infectious as well as non-infectious HIV-1 induced IFN-α in pDCs
via TLR7 signaling and consecutive involvement of autophagy processes. 500
ng/ml p24 antigen were used for stimulation of 1 x 105 pDCs for 16 h. About 6500
pg/ml IFN-α were measured in the supernatant of likewise stimulated pDCs in this
publication [181]. We used 5 ng/ml p24 antigen, a much lower concentration that is
closer to actual concentrations in primary infection, to stimulate pDCs [110]. To
explain the high IFN-α amounts measured in our co-cultures of HIV-1-infected
CD4+ T cells with pDCs, it is important to remark again that pDCs are able to
recognize HIV-1-infected lymphocytes. This process depends on interaction with
CD4 on pDCs and on endocytotic mechanisms [153] finally leading to signaling via
TLR7 [106]. It is known that pDCs produce markedly increased and substantial
amounts of IFN-α on encounter with these HIV-1+ CD4+ T cells [153]. Overall this
shows that HIV-infected T cells are much more potent stimuli for pDCs than HIV
virions alone [106]. In addition, others suppose that 300-1000 ng/ml p24 are
needed to induce substantial IFN-α secretion by pDCs [52]. Consequently our
results for IFN-α secretion by pDCs alone or after co-culture with HIV-1-infected
CD4+ T cells are plausible and in line with published data.
62
As pDCs also interact with other immune cells via their surface molecules, surface
molecule expression was analyzed on pDCs incubated with the three different
herpesviruses. As a pre-requisite for this analysis, it has to be taken into account
that IL-3 alone has the potential to maturate pDCs and therefore influences
surface molecule expression. Maturation of pDCs with IL-3 leads to upregulation of
co-stimulatory molecules CD80 and CD86 on pDCs as well as to upregulation of
HLA-DR [91], the maturation marker CD83 [44] and the intercellular adhesion
molecule CD54 [64]. This fits our data because when pDCs were incubated with
VZV [47], UV-CMV or EBV in addition to IL-3 neither any modulation of the high
expression of HLA-DR nor of maturation markers on IL-3-pDCs were seen.
Previous research shows that pDCs incubated with CMV in IL-3-supplemented
medium could upregulate HLA-DR [99], CD 40 and CD80 [154], but the basically
low expression of CD83 was not affected [99]. Another study demonstrated for
pDCs incubated without IL-3 that CMV upregulated HLA-DR and the maturation
marker CD83 on the surface, but not CD80 and CD86 [171]. The differing results
compared to ours may be due to the fact that we used UV-CMV instead of
replicable CMV and that we used IL-3 as an important survival factor for pDCs in
vitro [64]. In one additional study UV-CMV did not alter surface molecule
expression on IL-3-pDCs [154], a result partly in line with our data.
Data on surface molecule regulation on pDCs by EBV are ambiguous and have
been tested with pDCs incubated without supplementation of IL-3 [50]. However
one has to take into account that the amounts of EBV applied and other
experimental settings are not mentioned and probably differed in these studies.
Two authors reported that co-stimulatory molecules such as CD40, CD80 and
CD86 as well as HLA-DR are rather upregulated on the surface of pDCs upon
challenge with EBV [50, 108]. In contrast, others found that EBV was not able to
maturate pDCs efficiently, as CD80 and CD86 were hardly increased on the cell
surface and pDCs secreted also less TNF-α [155]. This is in line with our data that
EBV did not enhance HLA-DR expression on IL-3-pDCs. In conclusion, we
suppose that pDCs rather display an immature phenotype in EBV infection.
In addition, we investigated two other important molecules for the interaction of
pDCs with T cells: HLA-ABC and CD54. VZV proved itself as a potent inducer of
HLA-ABC expression on pDCs [47]. Even high volume dilutions induced HLA-ABC
expression on pDCs. UV-CMV was also a potent inducer of HLA-ABC on pDCs.
63
By contrast, EBV did not induce HLA-ABC. An upregulation of HLA-ABC might
enhance interaction of VZV- or UV-CMV-stimulated pDCs with cytotoxic CD8+ T
cells. Moreover, this interaction may be supported in a particularly efficient way as
pDCs are equipped with intracellular pre-formed stores of HLA-DR and of HLAABC [38]. HLA-ABC is localized within the recycling endosomal compartment.
Exogenous viral antigen is taken up and loaded on HLA-ABC in a proteasomeindependent manner and without a detour through the cytosol. Furthermore, HLAABC is upregulated on the surface of pDCs within 30 min upon stimulation with
influenza virus. Antigen loaded HLA-ABC is then able to mediate crosspresentation of exogenous antigen to CD8+ T cells. Therefore cross-presentation
enables pDCs to induce a very rapid adaptive immune response to viral agents
besides the prompt anti-viral effects of type I IFNs [38].
VZV was also able to relevantly enhance CD54 expression on pDCs [47] whereas
EBV only minimally induced CD54 expression. CD54, otherwise named
intercellular adhesion molecule 1 (ICAM-1), interacts with leukocyte functionassociated antigen (LFA-1) that is expressed for example on lymphocytes. The
interaction of CD54 with LFA-1 is important for T cell activation. Recently, it was
found that CD54 can recruit HLA-ABC molecules to the cell surface, further
facilitating the activation of CD8+ T cells [101]. In line with our results
demonstrating enhanced expression of CD54 upon stimulation with VZV and EBV,
one study in mice showed that pDCs upregulated CD54 expression upon
stimulation with TLR7 or TLR9 ligands. CD54 on pDCs contributed thereafter to
the proliferation and auto-Ab secretion of auto-reactive B cells by interacting with
LFA-1 on B cells [41]. Enhanced expression of CD54 on the surface of pDCs
might consequently contribute to the induction of an adaptive immune response
via interaction with B cells and T cells. Overall in our study pDCs reacted with a
more or less immunostimulatory phenotype to the herpesviruses tested: VZV
induced a robust immunostimulatory phenotype with increased expression of both
HLA-ABC and CD54 on IL-3-pDCs, whereas CMV only induced HLA-ABC and
EBV only minimally induced CD54 on the surface of IL-3-pDCs. We suppose that
pDCs might thereby stimulate a potent adaptive immune response in VZV infection
and to a smaller extent in CMV infection. One should certainly not ignore the
diverse mechanisms herpesviruses have developed to undermine the immune
response, especially the antigen presentation machinery [58, 83]. Nevertheless
64
this point will not be discussed here as we performed only short term cell culture of
16 h and the herpesvirus stimuli except for EBV are probably not permissively
infecting peripheral blood pDCs. The impact of soluble factors in the preparation of
UV-CMV and EBV cannot be ruled out as we did not check for the influence of the
supernatant controls on pDC surface molecule expression.
In this study, we did not analyze surface molecule regulation by HIV-1. Others
found that HIV-1 did not modulate the expression of co-stimulatory molecules and
CD83 on pDCs incubated with IL-3 [153]. A more recent report found that HIV-1
was sensed in early endosomes, like CpG A, inducing a rather immature
phenotype of pDCs [136]. But co-culture of pDCs with phytohemagglutinin (PHA)activated CD4+ T cells or with HIV-1-infected CD4+ T cells led to maturation of
pDCs. Incubation with HIV-infected T cells additionally induced upregulation of
CCR7 on pDCs mediating possible migration to lymph nodes [153]. Regarding
IFN-α production as well, T cell-based HIV seems to be a more potent stimulus for
the activation of pDCs finally leading to the induction of an immune response
[153].
4.4 The role of plasmacytoid dendritic cells in anti-viral immunity and
viral pathogenicity
The role of pDCs in viral infections in the human system is to date not entirely
clear. In our study we investigated the herpesviruses VZV, CMV and EBV and the
retrovirus HIV-1. Here we focus on discussing data concerning the role of pDCs
during viral infections with VZV, EBV, CMV and HIV-1.
To date, the role of pDCs in VZV infection has not been extensively investigated.
In a single case report of a 23-year-old patient with acute varicella infection
peripheral blood pDCs were reduced to 0.01% of PBMCs compared to the levels
of four controls with 0.48% pDCs [59]. Additionally, influx of pDCs into the dermis
was detected in infected skin biopsies. Three weeks later a percentage of 0.68%
pDCs was measured in the patient’s blood [59]. Another study expanded these
findings. In cutaneous biopsies of patients suffering from primary VZV infection or
herpes zoster, pDCs were shown to immigrate into the infected dermis and to a
lower extent into the epidermis in contrast to normal uninfected skin [81]. The loss
of pDCs in peripheral blood might therefore result from relocation of pDCs into the
65
infected skin where pDCs may contribute to an efficient immune response by
antigen uptake and other characteristic pDC functions. Whether VZV inhibits IFN-α
secretion by pDCs [81] or induces some IFN-α, like in our hands, remains elusive.
But the upregulation of CD54 and HLA-ABC on the cell surface of pDCs as
described in our results rather implicates the induction of a protective immune
response by VZV. The reduction of GrB production by pDCs upon VZV stimulation
and the induction of T cell proliferation by VZV-pDCs in the MLRs underline this
thesis. The VZV vaccine is clinically quite effective and was shown to induce
equally potent T cell responses as the natural infection. When VZV+ patients got
vaccinated, the numbers of T cells reacting to VZV even increased in these
individuals [5], a fact in line with our results of GrB inhibition in pDCs by VZV
licencing T cell proliferation.
The role of pDCs in the immune response against CMV infections was
investigated in several publications. The observations of one group that CMVstimulated pDCs activated B cells and additional T cell help or T cell-derived IL-2
allowed Ab production by B cells [171] are in line with the immunostimulatory
function of UV-CMV-stimulated pDCs in our MLRs. But in contrast to our MLR
results others found that IL-3-pDCs stimulated with UV-CMV did not alter CD4+ T
cell proliferation in comparison to mock-infected pDCs [154]. In their hands, IL-3pDCs stimulated with active CMV even inhibited proliferation of CD4+ T cells in
MLRs, possibly via a soluble factor in the supernatant of CMV-infected pDCs
[154]. Of note, pDCs were stimulated with CMV MOI 50 what might account for the
diverging results as well as the different pDC:T cell ratios. Another group showed
as well that pDCs stimulated with active CMV or UV-CMV limited the proliferation
of allogeneic CD4+ and CD8+ T cells [171] most likely due to diverging
experimental conditions. One reason for the differences to our results might be the
general pre-incubation of pDCs in IL-3-supplemented medium in our experiments.
Lack of IL-3 may explain the failure of CMV-stimulated pDCs to upregulate costimulatory molecules observed by others [171]. Incubation with IL-3 upregulates
per se CD80 and CD86 expression on pDCs and we did not observe a modulating
effect of UV-CMV on the expression of these molecules compared to stimulation
with IL-3 alone. Therefore the basic incubation of pDCs with IL-3 may have
provided sufficient pDC surface molecule expression for pDC-T cell interaction so
that other factors produced by pDCs and regulated by CMV like for example GrB
66
were able to further modulate the pDC proliferation-inducing capacity in our
experiments; a fact that might be responsible for the diverging results. One has to
take in account that IL-3 might play an important role for pDC survival not only in
vitro [64], but also in vivo and might thereby influence the pDC response in vivo as
well. On the other hand the difference in the MOI applied may have had diverging
effects as well as the different pDC:T cell ratios. Overall, in contrast to our data,
literature rather indicates a low allogeneic-stimulation potential of CMV-pDCs in
MLRs. Moreover, the activation status of CMV seems to play an important role for
pDC activation. Active CMV should be used in further experiments as relevant
differences for the interaction of UV-CMV or active CMV with pDCs are present in
literature.
The interaction of human CMV with the immune system and pDCs is quite
complex as the herpesvirus is well adapted to its human host and has developed a
lot of strategies to undermine the host’s immune response with generally low
pathogenicity [112, 113]. Mechanisms taking advantage of the host’s immune
response have been described for CMV such as promotion of its own replication
and reactivation by provoking an inflammatory immune status via TNF-α and IFN-γ
induction [25]: CMV-infected pDCs induced CD69 on and IFN-γ and TNF-α in NK
cells. On the other hand, CMV-infected pDCs did not stimulate cytotoxic activity of
NK cells. The authors hypothesize that CMV might thereby evade an innate
immune response as NK cells were impaired in their killing function [25].
Moreover, human CMV produces an IL-10 homologue (cmvIL-10) that diminishes
IFN secretion upon TLR9 stimulation in pDCs up to 75% [28]. The CMV IL-10
homologue displayed the same inhibitory effects as recombinant human IL-10
(hIL-10) [28] and is a late antigen [27]. Still, the induction of maturation by TLR
agonists was not hampered by addition of cmvIL-10 or hIL-10 [28]. The expression
levels of HLA-DR and co-stimulatory molecules on pDC surface remained the
same and as a consequence antigen presentation capacities of pDCs should not
be affected [28]. To sum up, in line with our data showing enhanced expression of
HLA-ABC on UV-CMV-pDCs, pDCs seem to represent potent antigen presenting
cells in the course of CMV infection independent of potential interfering viral
factors. But the authors hypothesize that cmvIL-10 might influence the interaction
of pDCs with other immune cells via inhibition of a potent IFN response [28]. As IL10 also decreases the viability of pDCs activated with CpG ODNs [43] and
67
because IFN-α itself is a survival factor for pDCs, the hypothesis arose that CMV
might try to decrease pDC viability by producing cmvIL-10 [28]. According to the
authors, CMV seems to hinder an efficient early immune response and the above
mentioned mechanisms might contribute to latency establishment of CMV [28].
This assumption is supported by the fact that pDC numbers in peripheral blood
were diminished in immunocompetent patients suffering from a symptomatic
primary mononucleosis-like CMV infection [172]. In conclusion, peripheral blood
pDCs might normally have a protective role in CMV infection as their reduction
was associated with symptomatic disease. PDCs could therefore be a factor
influencing the wide range of pathogenicity of CMV infections. The reason why
CMV infection is mostly asymptomatic in immunocompetent individuals remains
elusive. One possible mechanism by which CMV promotes a sufficient anti-viral
immune response is suppression of pDC-derived GrB as shown in our data.
Moreover, certain individual factors such as biological age determine the outcome
of an anti-CMV immune response. It was observed that IFN-α derived from pDCs
stimulated with CMV could reduce telomerase activity in and induce loss of the costimulatory molecules CD27 and CD28 on CD4+ CMV-specific T memory cells
[53]. The consequence might be a loss of these CMV-specific memory cells. The
authors suggest that this process could take place in T cell areas of lymph nodes.
CMV reactivation and consequent IFN-α production by pDCs could thereby affect
longevity of other memory T cells as well. To sum up, these results show that the
interaction of CMV with pDCs might influence the composition of adaptive immune
cells and further underlines the relevance of the often asymptomatic CMV infection
for carriers [53].
Overall the effects of CMV on the innate and adaptive immune system and
especially on pDCs are diverse and need to be further elucidated as literature on
CMV effects on immunity is quite controversial and the interactions are quite
complex. We suppose that pDCs could play a protective role in the immune
response against CMV. Based on our results we assume that suppressed GrB
expression in peripheral blood pDCs might favor a protective T cell response
against CMV infections and may keep CMV in check in CMV+ individuals at least
in middle-aged humans. But as literature provides more evidence for an inhibiting
effect of pDCs on T cell proliferation in CMV infection it would be interesting to
68
investigate
the
effects
of
active
CMV
on
GrB
production
and
the
immunostimulatory potential of pDCs.
Next, the role of pDCs in EBV infection shall be analyzed. It is still questioned
whether EBV can infect pDCs or not. Some authors report that pDCs secreted
moderate to low amounts of IFN-α in a replication-independent manner and
upregulated co-stimulatory molecules on their surface upon EBV stimulation [50,
144]. Others showed permissiveness of pDCs to EBV and report that EBV induced
high amounts of IFN-α whereas UV-inactivated EBV was only able to induce very
little IFN-α in pDCs which was interpreted as a need for viral replication in the
cytosol [155]. Autophagy seemed to be involved [155]. In addition, EBV induced
only minor maturation in pDCs. Even the inducing effect of CpG C on pDC
maturation was diminished by EBV [155]. These results are in line with our data
indicating that CpG B-pDCs additionally incubated with EBV displayed a lower
allogeneic stimulation potential in MLRs with T cells than pDCs incubated with
CpG B alone. Moreover, pDCs stimulated with EBV expressed the regulatory
molecules inducible T cell co-stimulatory-ligand (ICOS-L) and B7 homolog 1 (B7H1) on their surface leading to a reduced allogeneic CD4+ T cell answer [155]. In
conclusion, these results imply that EBV is able to infect pDCs and to impair their
potential to induce potent CD4+ T cell responses [155]. This effect might be
mediated by the enhanced expression of inhibitory molecules such as ICOS-L [85]
and B7-H1 [20] on the surface of pDCs at the expense of a sufficient expression of
co-stimulatory molecules. This interaction with pDCs might favor the establishment
of viral persistence [155].
Taken together, literature provides controversial data on EBV-incubated pDCs,
either showing high or low IFN-α production, probably depending on the specific
experimental settings. Most data point to a rather immunoregulatory phenotype of
pDCs after EBV stimulation, including our own results showing a rather low
allogeneic stimulation potential of EBV-pDCs.
Nonetheless, it is well established that cellular immunity is decisive for the control
of EBV infection. Consequently the induction of Ag-specific T cells is essential
besides the activation of NK cells [129]. In contrast to the rather regulatory role of
pDCs in the course of EBV infection described above, another group found that
pDCs stimulated with EBV induced IFN-γ in CD3+ T cells in a TLR9-dependent
69
manner and additionally activated NK cells. Both processes were based on cell
contact of pDCs with NK cells or T cells [108].
To summarize, contradictory data exist concerning the immunostimulatory
potential of pDCs activated by EBV. Our results indicate a low immunostimulatory
potential of EBV-activated pDCs going along with high GrB secretion levels.
Interestingly, one group reported that GrB levels were elevated in the blood serum
of patients suffering from EBV infection in different disease stages. GrB levels
derived from 14 patients ranged from 1-4000 pg/ml with a median of 60 pg/ml.
Despite these high inter-individual differences in GrB levels, the tendency was
derivable, that high GrB levels were detected in patients suffering from acute
mononucleosis and that these high GrB levels went down to normal levels after
the acute phase of the infection. In this study the median GrB level of 54 healthy
control subjects was measured with 11.5 pg/ml [161].
In conclusion, EBV can induce a rather immature phenotype in pDCs thereby
hampering an efficient EBV immune response in particular after higher GrB
induction. It remains to be determined to what extent high GrB secretion by pDCs
stimulated with EBV is decisive for the clinical presentation of EBV infection.
Factors produced by EBV upon infection such as vIL-10 may play a modulating
role. Moreover, EBV could try to limit T cell responses not only by inducing high
GrB levels in pDCs but also by enhancing the expression of other
immunoregulatory molecules such as ICOS-L and B7-H1 on the surface of pDCs.
Overall, it will be interesting to further elucidate the mechanisms responsible for
the variable pathogenicity of EBV infection. One mechanism might be GrB
regulation in pDCs as higher GrB levels may go along with higher pathogenicity of
EBV infection.
The most important features of chronic HIV infection and progression to AIDS are
dysfunction, essentially the progressive loss of CD4+ T cells, but also
hyperactivation
of
the
host’s
immune
system.
The
role
of
pDCs
in
immunopathogenesis of HIV infection is complex and their potential to secrete
high amounts of IFN-α is regarded as ambiguous concerning both a protective and
a detrimental potential of this cytokine in the course of HIV infection.
Lots of data are in favor of a rather protective role of pDCs in the course of HIV
infection:
70
First, there is a loss of pDCs in the peripheral blood of patients suffering from
advanced HIV-1 infection. Furthermore decreased pDC numbers were associated
with elevated viral load and an increased appearance of opportunistic infections.
These observations were seen as a hint for a protective role of pDCs in the course
of HIV infection [138, 160]. Furthermore it was shown that pDC numbers were
already decreased in the peripheral blood in early, primary HIV-1 infection [80,
151]. In some studies, the decline of pDCs in peripheral blood was accompanied
by the accumulation of pDCs in lymphoid tissues, perhaps representing a
redistribution of pDCs. CCL19 interacted with CCR7 that was upregulated on
pDCs in HIV infection [55] and mediated migration of pDCs to secondary lymphoid
tissues [51] where they might undergo apoptosis as described for pDCs in simian
immunodeficiency virus (SIV) infection [21]. Accumulated pDCs in lymph nodes
were immature [40] and secreted high amounts of IFN-α [105]. PDCs also
accumulated in the spleen of viremic patients but there, surprisingly, they did not
represent the main source of IFN-α [132]. Other reports found that classical DCs
as well as pDCs were lost in lymph nodes of HIV patients in the chronic phase
[13]. In another publication, pDCs were preserved in lymph nodes from patients in
different disease stages and pDC numbers were comparable to those in lymph
nodes of healthy controls [103], further contradicting the thesis of pDC loss due to
redistribution. Another explanation for pDC loss might be that HIV-1 itself induced
direct cell death in pDCs after fusion of the virion with pDCs [124]. The reason for
the pDC loss in HIV infection consequently remains elusive.
Besides, pDC function seems to be impaired in HIV-infected individuals: PBMCs
from HIV-1-infected patients stimulated in vitro with TLR7 agonists secreted less
IFN-α than PBMCs from healthy individuals. Intracellular staining revealed that
less pDCs produced IFN-α [119]. HIV gp120 was shown to bind to BDCA-2 on
pDCs and inhibited IFN-α, TNF-α and IL-6 secretion, NK cell stimulating capacity
as well as CD83 expression by pDCs upon stimulation with a TLR9 agonist but not
upon stimulation with a TLR7 agonist [118]. These results imply that HIV interferes
also with the capacity of pDCs to respond to TLR9 signals such as other viral
agents or bacteria [118] possibly facilitating opportunistic infections. Several
authors showed that the crosstalk of pDCs with NK cells was affected [34, 145]
and hence activation of NK cells by pDCs was impaired in HIV-1 infection [11].
One factor might be HIV viral protein R (Vpr) that was shown to inhibit type I IFN
71
production by pDCs [79]. What is more, HAART was not able to completely rescue
pDC function and numbers, neither in adults [29, 49] nor in children [180]. The
ability of HIV to hamper pDC functions is reflected in our data showing low IFN-α
secretion by pDCs stimulated with HIV-1 and a low allogeneic stimulation potential
of HIV-1-pDCs in MLRs with CD4+ T cells.
On the contrary, a protective role of pDCs in HIV infection in some cases is
underlined by the fact that HIV-1 maturated pDCs and enabled pDCs to activate
and maturate bystander myeloid DCs and CD4+ T cells [55]. In addition, HIVantigen coupled to a lipopeptide or derived from HIV-infected apoptotic cells could
be cross-presented by pDCs to CD8+ T cells [78]. Consequently pDCs seem to be
able to induce adaptive immune responses against HIV under certain conditions.
In elite controllers, representing HIV patients able to suppress viral replication
under detectable levels for long time periods, a recent study found retained pDC
numbers and IFN-α-producing capacity. Moreover pDCs were able to diminish
viral replication and could bring about apoptosis of T cells [116]. PDCs from
healthy individuals stimulated with HIV-1+ CD4+ T cells secreted IFN-α and were
able to suppress viral replication in these T cells in an IFN-α-dependent manner
[153]. CpG A-stimulated pDCs from HIV-1-infected patients limited viral replication
in autologous CD4+ T cells in a partially IFN-α-dependent manner. Interestingly,
even unstimulated pDCs from HIV-1-infected patients with low viral load were able
to limit viral replication in autologous CD4+ T cells in a cell contact-dependent
manner [124] further supporting a protective role of pDCs in HIV. Thus in some
patients pDCs can help to control HIV infection.
But there is also evidence that HIV abuses pDCs for its own advantage,
manipulating pDC functions in a rather detrimental and pathogenic manner,
especially in chronic disease stages. Currently immune hyperactivation is seen as
one of the major causes of HIV progression and pathogenicity. Surprisingly
enough, HIV infection in women develops faster into AIDS than in men relative to
viral load [122]. Differences in the response of pDCs to TLR7 stimuli such as
HIV-1 have been detected as female pDCs responded with higher IFN-α
production [122]. Furthermore CD8+ T cells showed a more activated phenotype in
women compared to men for the same viral load. Overall a higher immune
activation seemed to contribute to faster disease progression in female HIV
72
patients [122], underlining the relevance of overactivation of the immune system in
HIV pathogenesis.
The exact role of IFN-α in HIV immunopathogenesis is quite controversial and
diverging results are reported. In one study peripheral blood pDCs from patients in
early stages of HIV infection were hyperresponsive to stimulation with a TLR7/8
agonist producing higher levels of diverse chemokines and cytokines, including
IFN-α, in contrast to pDCs from uninfected controls [151]. Another study presented
data that pDCs expressed less IFN-α per cell in early HIV infection [80]. The
differences might be due to use of different methods. Another group showed that
PBMCs from HIV-infected patients displayed a markedly higher expression profile
of IFN-α compared to PBMCs from healthy individuals [103]. Especially the
subtype IFN-α2 was increased in PBMCs from patients with HIV infection inversely
correlating with CD4+ T cell counts [104]. On the other hand, lymphoid tissue
mononuclear cells did not present higher IFN-α messenger (m) RNA levels in HIV
patients [103]. Therefore, IFN-α levels in HIV infection show great variability, what
may reflect different stages of infection. Our data support the assumption that poor
IFN-α induction correlates with generally high viral pathogenicity as is the case in
typical HIV infection.
In conclusion the role of pDCs in HIV-1 infection still needs to be further elucidated
and clarified. On one hand, pDCs might control viral replication in the early
infection phase via IFN-α secretion and might induce a protective adaptive
immune response against HIV-1. On the other hand, IFN-secreting pDCs might
contribute to immune hyperactivation in lymph nodes leading to the loss of T cells
in the chronic phase of infection. Another possibility is that pDCs represent a
tolerogenic population limiting T cell proliferation that is lost in chronic infection
and cannot limit the overactivation of the immune system anymore. As described
in the following section, GrB might contribute to this regulatory function with
limiting T cell expansion upon encounter with virus. Via inhibition of uncontrolled T
cell proliferation GrB-secreting pDCs might potentially protect T cells from being
killed.
73
4.5 Granzyme B and tolerogenic plasmacytoid dendritic cells in antiviral immunity
PDC-derived GrB has been shown to limit T cell proliferation in a cell contactdependent but perforin-independent manner [87]. Anti-viral vaccines against
ssRNA viruses inhibited GrB in pDCs and were therefore able to induce CD4+ T
cell proliferation [47]. Concordantly to the results of our work that VZV and UVCMV inhibit GrB production and secretion by pDCs, proliferation of CD4+ T cells
was enhanced when pDCs were pre-stimulated with VZV or CMV. However, EBV
enhanced GrB production and secretion by pDCs. In MLR with CD4+ T cells and
EBV-pre-treated pDCs, proliferation of allogeneic T cells was on the same low
level whether pDCs were pre-incubated only with IL-3 or additionally with EBV in
increasing volume dilutions. When pDCs were pre-incubated with CpG B and
EBV, EBV even slightly decreased the proliferation-inducing potential of CpG BpDCs. This finding was accompanied by a slight increase in GrB production in
pDCs pre-incubated with CpG B and EBV compared to CpG B stimulation alone
(data not shown). Likewise HIV-1, another positive regulator for GrB secretion by
pDCs, did not further modulate the effect of IL-3 alone-pre-incubated pDCs on
allogeneic T cell proliferation. For UV-CMV, EBV and HIV-1 only one independent
MLR was performed although with different donors. Consequently, it is difficult to
derive a real statement from these results. But the different direction of GrB
regulation in pDCs by viruses seems to inversely correlate with the effect of virusstimulated pDCs on CD4+ T cell proliferation in MLRs.
Therefore one has to discuss which role pDC-derived GrB might play during viral
infections. We hypothesize that pDC-derived GrB has a regulatory function. This is
in line with reports that pDCs exert also other immunoregulatory functions. IL-3 +
CD40L-stimulated pDCs could prime naïve CD4+ [85] and CD8+ T cells [60] to
become regulatory T cells. PDCs incubated with different classes of CpG ODNs
primed CD4+ Tregs [85, 128]. The mechanisms behind these tolerogenic functions
might be additional immunoregulatory molecules. CpG ODN-stimulated pDCs
expressed IDO and were able to induce inducible Tregs via this mechanism [30].
In another study pDCs expressed ICOS-L upon maturation and induced IL-10
expressing regulatory CD4+ T cells [85]. Interactions like that have been also
described for natural viral ligands. For example, HIV enhanced IDO expression on
74
pDCs in a TLR7-dependent manner [117]. These pDCs were able to induce the
generation of suppressive T cells from naïve allogeneic CD4+ T cells. These Tregs
inhibited CD4+ T cell proliferation and classical DC maturation. The authors
consequently attributed an immunoregulatory function to pDCs in the course of
HIV infection [117]. Other authors support this finding. In their hands HIV-1 also
induced IDO in pDCs that inhibited the proliferation of both CD4+ and CD8+ T cells
[17, 18]. In allogeneic MLRs with pDCs derived from HIV-infected individuals pDCs
showed equal stimulation capacity compared to pDCs from healthy subjects. But
pDCs from highly viremic, acutely infected patients were not as efficient in
stimulation of allogeneic T cell proliferation [151]. Another study supported this
finding by showing a decreased potential of pDCs from HIV-infected individuals to
promote proliferation of allogeneic HIV-, enriched T cells [42] maybe representing
a protective mechanism against uncontrolled T cell activation mediated by pDCs.
Summing up, our data showing elevated GrB secretion by pDCs upon challenge
with HIV-1 and a limitation of CD4+ T cell proliferation in MLRs promote the
hypothesis of pDCs being a tolerogenic population in HIV-1 infection that may
hamper efficient T cell responses but can also limit hyperactivation of the immune
system and concomitantly viral spread.
Apart from pDCs incubated with HIV, pDCs stimulated with EBV can also express
the immunoregulatory molecule ICOS-L on their cell surface [155]. Moreover,
pDCs stimulated with HSV-1 induce regulatory IL-10 and IFN-γ-producing CD4+ T
cells via IFN-α. Besides their suppressive function these T cells acquired cytotoxic
functions by expressing GrB and perforin. By inducing regulatory T cells, virusstimulated pDCs might not only contribute to a contraction of the immune
response but as a possible negative side effect also to the establishment of
persistent viral infections [93]. PDCs stimulated with CMV induced IL-10 secretion
in CD4+ memory T cells that were consecutively also able to produce IFN-γ. Such
stimulated T cells were able to suppress allogeneic T cell proliferation and
proliferated well themselves. These results implicate that pDCs are even able to
control memory T cell responses. The theory of the authors is that pDCs allow Agspecific memory T cells to control the expansion of naïve T cells at sites of reinfection via IL-10 in favor of the expansion of Ag-specific memory T cells in early
times of infection [100]. To sum up, pDCs seem to be able to orchestrate a lot of
tolerogenic T cell responses especially in the course of viral infections. Overall
75
these tolerogenic properties might limit detrimental immune system overactivation
and might guide specific T cell responses. The limitation of an immune response
might on the other hand also facilitate the establishment of latent infections.
We suppose a regulatory role of pDC-derived GrB that represents one possible
effector molecule produced by pDCs to orchestrate immune responses.
Of note, in contrast to our hypothesis of tolerogenic pDC-derived GrB, elevated
GrB levels in the plasma are seen by some authors as a sign of immune
activation. In their opinion GrB might promote inflammatory disease progression
[4] and an elevation of GrB in the extracellular space might represent an overactivated immune system [1] which would be in line with the theory of detrimental
immune system hyperactivation as shown for HIV infection. The exact role of
pDCs in viral immunity needs further elucidation and especially their regulatory
potential that might be in part mediated by GrB should be further analyzed.
To sum up, our hypothesis that pDC-derived GrB is virus-specifically regulated
was confirmed. T cell proliferation was modulated according to GrB levels
produced by pDCs. The suppression of pDC-derived GrB by VZV and UV-CMV
might facilitate the induction of a potent T cell response. EBV might profit from GrB
elevation to hamper a rapid adaptive immune response. In the course of HIV-1
infection high GrB levels could represent a possible regulatory mechanism by
which pDCs limit uncontrolled T cell proliferation. On the other hand, HIV-induced
GrB induction may contribute to a failure of a HIV immune response which
correlates with severe disease in most untreated individuals. In conclusion, pDCderived GrB seems to represent a tightly regulated molecule in anti-viral immunity.
GrB produced by pDCs may inhibit uncontrolled proliferation of T cells, a favorable
effect, but beyond that can lead to unfavorable immune suppression.
76
4.6 Outlook
In the face of our data presented in this work and with regard to the discussed
literature many questions remain to better understand the role of pDCs in anti-viral
immunity. In the future it would be first of all interesting to further investigate the
signaling pathways of different viral agents in pDCs leading to GrB production.
This could be addressed with the help of specific TLR antagonists. The role of
IL-10 that might be included in viral preparations but could also be produced by
pDCs and infected bystander cells should be evaluated. One factor that might
additionally influence our results in pDC cultures as well as in MLRs might be
earlier viral infections reflected by the serostatus of the blood donors which was
unknown. It would be interesting to analyze the serostatus of blood donors and to
investigate whether this parameter has an impact on pDC responses or MLRs.
Furthermore, the phenotype of CD4+ T cells co-cultured with virus-pre-treated
pDCs should be specified. In particular the expression of regulatory molecules
such as IDO and ICOS-L on pDCs should be measured. Besides, the effect of
virus-stimulated pDCs on CD8+ T cells should be tested in MLRs. In addition, the
exact mechanism by which GrB limits T cell proliferation needs to be elucidated
beyond the finding of cleavage of the TCR ζ-chain [47].
77
5 Summary
Plasmacytoid dendritic cells (pDCs) are a rare but specialized immune cell subset
that represents an important link between innate and adaptive immunity. The most
known function of pDCs is their capability to respond with high amounts of type I
interferons (IFNs) to viral stimuli. Through the expression of Toll-like receptor
(TLR)7 and TLR9 pDCs are well equipped to respond to single-stranded
ribonucleic acid (ssRNA) viruses such as human immunodeficiency virus 1 (HIV1) and double-stranded deoxyribonucleic acid (dsDNA) viruses such as the
herpesviruses varicella-zoster virus (VZV), cytomegalovirus (CMV) and EpsteinBarr virus (EBV). Despite their immunogenic functions, especially in anti-viral
immunity, pDCs are also known to exert tolerogenic functions. PDCs were found
to express the serine protease granzyme B (GrB) in the past only known as the
apoptosis-inducing effector protease of natural killer cells and cytotoxic T
lymphocytes. Recent years of research have revealed new sources of GrB, such
as pDCs, and have attributed many new functions to this molecule. PDC-derived
GrB was shown to suppress T cell proliferation indicating that a regulatory
mechanism is mediated by this molecule.
In this study we evaluated the effects of diverse viral stimuli on human pDCderived GrB, the phenotype and function of human pDCs. The viruses investigated
establish latency or chronic infection and adaptive immunity plays an important
role for virus control. We measured GrB production and secretion as well as
surface molecule expression and IFN-α secretion by pDCs upon stimulation with
vaccine-derived live attenuated VZV, ultraviolet light (UV)-inactivated CMV,
replicable EBV and HIV-1 with flow cytometry and enzyme-linked immunosorbent
assays (ELISAs). Furthermore we analyzed the influence of virus-stimulated pDCs
on proliferation of co-incubated carboxyfluorescein diacetate succinimidyl ester
(CFSE)-labelled Cluster of differentiation (CD)4+ T cells in mixed lymphocyte
reactions (MLRs). PDCs and T cells were isolated with magnetic beads from buffy
coats or from fresh blood of healthy donors.
GrB production and secretion were differentially regulated by the viral stimuli. VZV
and CMV diminished GrB production in pDCs whereas EBV and HIV-1 increased
GrB secretion indicating that GrB expression by pDCs is virus-specifically
regulated. Robust IFN-α production was only observed for VZV and CMV.
78
In contrast, EBV and HIV-1 induced only very low levels of IFN-α. On the contrary,
HIV-1-infected T cells led to relevant IFN-α secretion by pDCs in pDC-T cell cocultures. The herpesviruses VZV and CMV, but not EBV and HIV-1 induced a
rather immunogenic phenotype in pDCs. CMV and VZV upregulated human
leukocyte antigen (HLA)-ABC, VZV additionally CD54, on the cell surface of pDCs
enabling better interaction with T cells and cross-presentation of viral antigens.
CMV- and VZV-pre-stimulated pDCs were able to enhance T cell proliferation in
MLRs whereas EBV and particularly HIV-1 failed to provoke significant T cell
proliferation. We conclude that the effective adaptive immune response of healthy
individuals to VZV might be supported by suppression of GrB in pDCs and the
induction of an immunostimulatory phenotype on pDCs. Similarly, in CMV
infection, peripheral blood pDCs could license CD4+ T cell responses by low GrB
secretion levels. Of note, we examined UV-inactivated CMV. In fact, literature
provides more evidence that pDCs stimulated with active CMV rather limit T cell
proliferation.
In our hands, EBV- and HIV-1-incubated pDCs did not enhance T cell proliferation,
an observation that might be partly dependent on GrB induction in pDCs. Via
elevation of pDC-derived GrB EBV might dampen a rapid protective immune
response allowing viral spread and establishment of latency supported by the
elsewhere published observation of relatively high GrB levels in active
mononucleosis. In HIV infection, there is evidence that elevated GrB secretion by
HIV-1-incubated
pDCs
might
inhibit
uncontrolled
T
cell
activation
and
hyperinflammation limiting HIV disease progression but on the other hand might
also represent one mechanism of progressive disease. Overall our MLR results
inversely correlated with the regulation of GrB in pDCs and support the finding that
pDC-derived GrB inhibits T cell proliferation. The data implicate that pDC-derived
GrB plays a role in anti-viral immunity as it is virus-specifically regulated. Virusspecific lower pDC-GrB levels might rather facilitate, high levels might limit T cell
responses. Further research is needed to elucidate the role of CD4+ T cell subsets
primed by virus-stimulated pDCs and the effect on CD8+ T cells. Our findings
contribute to further understand the interaction of viruses with pDCs. They show
that pDC-derived GrB is tightly regulated by different viruses and that pDC
regulation is worth further investigation.
79
6 References
1.
Afonina, IS, Cullen, SP, and Martin, SJ: Cytotoxic and non-cytotoxic roles of
the CTL/NK protease granzyme B. Immunol Rev 235: 105-116 (2010)
2.
Akira, S and Takeda, K: Toll-like receptor signalling. Nat Rev Immunol 4:
499-511 (2004)
3.
Andrade, F: Non-cytotoxic antiviral activities of granzymes in the context of
the immune antiviral state. Immunol Rev 235: 128-146 (2010)
4.
Anthony, DA, Andrews, DM, Watt, SV, Trapani, JA, and Smyth, MJ:
Functional dissection of the granzyme family: cell death and inflammation.
Immunol Rev 235: 73-92 (2010)
5.
Arvin, AM: Cell-mediated immunity to varicella-zoster virus. J Infect Dis 166
Suppl 1: S35-41 (1992)
6.
Asselin-Paturel, C, Boonstra, A, Dalod, M, Durand, I, Yessaad, N, DezutterDambuyant, C, Vicari, A, O'Garra, A, Biron, C, Briere, F, and Trinchieri, G:
Mouse type I IFN-producing cells are immature APCs with plasmacytoid
morphology. Nat Immunol 2: 1144-1150 (2001)
7.
Bailey-Bucktrout, SL, Caulkins, SC, Goings, G, Fischer, JA, Dzionek, A,
and Miller, SD: Cutting edge: central nervous system plasmacytoid dendritic
cells
regulate
the
severity
of
relapsing
experimental autoimmune
encephalomyelitis. J Immunol 180: 6457-6461 (2008)
8.
Barry, M, Heibein, JA, Pinkoski, MJ, Lee, SF, Moyer, RW, Green, DR, and
Bleackley, RC: Granzyme B short-circuits the need for caspase 8 activity
during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving
Bid. Mol Cell Biol 20: 3781-3794 (2000)
9.
Bauer, S, Kirschning, CJ, Hacker, H, Redecke, V, Hausmann, S, Akira, S,
Wagner, H, and Lipford, GB: Human TLR9 confers responsiveness to
bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad
Sci U S A 98: 9237-9242 (2001)
80
10.
Beignon, AS, McKenna, K, Skoberne, M, Manches, O, DaSilva, I,
Kavanagh, DG, Larsson, M, Gorelick, RJ, Lifson, JD, and Bhardwaj, N:
Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like
receptor-viral RNA interactions. J Clin Invest 115: 3265-3275 (2005)
11.
Benlahrech, A, Gotch, F, Kelleher, P, and Patterson, S: Loss of NK
stimulatory capacity by plasmacytoid and monocyte-derived DC but not
myeloid DC in HIV-1 infected patients. PLoS One 6: e17525 (2011)
12.
Berthou, C, Marolleau, JP, Lafaurie, C, Soulie, A, Dal Cortivo, L, Bourge,
JF, Benbunan, M, and Sasportes, M: Granzyme B and perforin lytic proteins
are expressed in CD34+ peripheral blood progenitor cells mobilized by
chemotherapy and granulocyte colony-stimulating factor. Blood 86: 35003506 (1995)
13.
Biancotto, A, Grivel, JC, Iglehart, SJ, Vanpouille, C, Lisco, A, Sieg, SF,
Debernardo, R, Garate, K, Rodriguez, B, Margolis, LB, and Lederman, MM:
Abnormal activation and cytokine spectra in lymph nodes of people
chronically infected with HIV-1. Blood 109: 4272-4279 (2007)
14.
Bjorck, P: Isolation and characterization of plasmacytoid dendritic cells from
Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated
mice. Blood 98: 3520-3526 (2001)
15.
Blom, B, Ho, S, Antonenko, S, and Liu, YJ: Generation of interferon alphaproducing predendritic cell (Pre-DC)2 from human CD34(+) hematopoietic
stem cells. J Exp Med 192: 1785-1796 (2000)
16.
Blomberg, S, Eloranta, ML, Cederblad, B, Nordlin, K, Alm, GV, and
Ronnblom, L: Presence of cutaneous interferon-alpha producing cells in
patients with systemic lupus erythematosus. Lupus 10: 484-490 (2001)
17.
Boasso, A, Herbeuval, JP, Hardy, AW, Anderson, SA, Dolan, MJ, Fuchs, D,
and Shearer, GM: HIV inhibits CD4+ T-cell proliferation by inducing
indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood 109:
3351-3359 (2007)
81
18.
Boasso, A, Hardy, AW, Anderson, SA, Dolan, MJ, and Shearer, GM: HIVinduced type I interferon and tryptophan catabolism drive T cell dysfunction
despite phenotypic activation. PLoS One 3: e2961 (2008)
19.
Bratke, K, Bottcher, B, Leeder, K, Schmidt, S, Kupper, M, Virchow, JC, Jr.,
and Luttmann, W: Increase in granzyme B+ lymphocytes and soluble
granzyme B in bronchoalveolar lavage of allergen challenged patients with
atopic asthma. Clin Exp Immunol 136: 542-548 (2004)
20.
Brown, JA, Dorfman, DM, Ma, FR, Sullivan, EL, Munoz, O, Wood, CR,
Greenfield, EA, and Freeman, GJ: Blockade of programmed death-1
ligands on dendritic cells enhances T cell activation and cytokine
production. J Immunol 170: 1257-1266 (2003)
21.
Brown, KN, Trichel, A, and Barratt-Boyes, SM: Parallel loss of myeloid and
plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS.
J Immunol 178: 6958-6967 (2007)
22.
Buzza, MS, Zamurs, L, Sun, J, Bird, CH, Smith, AI, Trapani, JA, Froelich,
CJ, Nice, EC, and Bird, PI: Extracellular matrix remodeling by human
granzyme B via cleavage of vitronectin, fibronectin, and laminin. J Biol
Chem 280: 23549-23558 (2005)
23.
Buzza, MS and Bird, PI: Extracellular granzymes: current perspectives. Biol
Chem 387: 827-837 (2006)
24.
Cavanagh, LL, Boyce, A, Smith, L, Padmanabha, J, Filgueira, L,
Pietschmann, P, and Thomas, R: Rheumatoid arthritis synovium contains
plasmacytoid dendritic cells. Arthritis Res Ther 7: R230-240 (2005)
25.
Cederarv, M, Soderberg-Naucler, C, and Odeberg, J: HCMV infection of
PDCs deviates the NK cell response into cytokine-producing cells unable to
perform cytotoxicity. Immunobiology 214: 331-341 (2009)
26.
Cella, M, Jarrossay, D, Facchetti, F, Alebardi, O, Nakajima, H,
Lanzavecchia, A, and Colonna, M: Plasmacytoid monocytes migrate to
inflamed lymph nodes and produce large amounts of type I interferon. Nat
Med 5: 919-923 (1999)
82
27.
Chang, WL, Baumgarth, N, Yu, D, and Barry, PA: Human cytomegalovirusencoded interleukin-10 homolog inhibits maturation of dendritic cells and
alters their functionality. J Virol 78: 8720-8731 (2004)
28.
Chang, WL, Barry, PA, Szubin, R, Wang, D, and Baumgarth, N: Human
cytomegalovirus suppresses type I interferon secretion by plasmacytoid
dendritic cells through its interleukin 10 homolog. Virology 390: 330-337
(2009)
29.
Chehimi, J, Campbell, DE, Azzoni, L, Bacheller, D, Papasavvas, E, Jerandi,
G, Mounzer, K, Kostman, J, Trinchieri, G, and Montaner, LJ: Persistent
decreases in blood plasmacytoid dendritic cell number and function despite
effective highly active antiretroviral therapy and increased blood myeloid
dendritic cells in HIV-infected individuals. J Immunol 168: 4796-4801 (2002)
30.
Chen, W, Liang, X, Peterson, AJ, Munn, DH, and Blazar, BR: The
indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid
dendritic cell-induced adaptive T regulatory cell generation. J Immunol 181:
5396-5404 (2008)
31.
Chowdhury, D and Lieberman, J: Death by a thousand cuts: granzyme
pathways of programmed cell death. Annu Rev Immunol 26: 389-420
(2008)
32.
Cohen, JI, Straus, SE, and Arvin, AM: Varizella-Zoster Virus Replication,
Pathogenesis, and Management. In: Knipe, DM and Howley, PM (Editors)
Fields Virology Fifth Edition, Vol. 2, Lippincott Williams & Wilkins,
Philadelphia, p. 2773-2818 (2007)
33.
Colonna, M and Cella, M: Crosspresentation: plasmacytoid dendritic cells
are in the business. Immunity 27: 419-421 (2007)
34.
Conry, SJ, Milkovich, KA, Yonkers, NL, Rodriguez, B, Bernstein, HB,
Asaad, R, Heinzel, FP, Tary-Lehmann, M, Lederman, MM, and Anthony,
DD: Impaired plasmacytoid dendritic cell (PDC)-NK cell activity in viremic
human immunodeficiency virus infection attributable to impairments in both
PDC and NK cell function. J Virol 83: 11175-11187 (2009)
83
35.
Crozat, K and Beutler, B: TLR7: A new sensor of viral infection. Proc Natl
Acad Sci U S A 101: 6835-6836 (2004)
36.
D'Amico, A and Wu, L: The early progenitors of mouse dendritic cells and
plasmacytoid predendritic cells are within the bone marrow hemopoietic
precursors expressing Flt3. J Exp Med 198: 293-303 (2003)
37.
Darmon, AJ, Nicholson, DW, and Bleackley, RC: Activation of the apoptotic
protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature 377: 446448 (1995)
38.
Di Pucchio, T, Chatterjee, B, Smed-Sorensen, A, Clayton, S, Palazzo, A,
Montes, M, Xue, Y, Mellman, I, Banchereau, J, and Connolly, JE: Direct
proteasome-independent
cross-presentation
of
viral
antigen
by
plasmacytoid dendritic cells on major histocompatibility complex class I. Nat
Immunol 9: 551-557 (2008)
39.
Diebold, SS, Kaisho, T, Hemmi, H, Akira, S, and Reis e Sousa, C: Innate
antiviral responses by means of TLR7-mediated recognition of singlestranded RNA. Science 303: 1529-1531 (2004)
40.
Dillon, SM, Robertson, KB, Pan, SC, Mawhinney, S, Meditz, AL, Folkvord,
JM, Connick, E, McCarter, MD, and Wilson, CC: Plasmacytoid and myeloid
dendritic cells with a partial activation phenotype accumulate in lymphoid
tissue during asymptomatic chronic HIV-1 infection. J Acquir Immune Defic
Syndr 48: 1-12 (2008)
41.
Ding, C, Cai, Y, Marroquin, J, Ildstad, ST, and Yan, J: Plasmacytoid
dendritic cells regulate autoreactive B cell activation via soluble factors and
in a cell-to-cell contact manner. J Immunol 183: 7140-7149 (2009)
42.
Donaghy, H, Gazzard, B, Gotch, F, and Patterson, S: Dysfunction and
infection of freshly isolated blood myeloid and plasmacytoid dendritic cells
in patients infected with HIV-1. Blood 101: 4505-4511 (2003)
43.
Duramad, O, Fearon, KL, Chan, JH, Kanzler, H, Marshall, JD, Coffman, RL,
and Barrat, FJ: IL-10 regulates plasmacytoid dendritic cell response to
84
CpG-containing immunostimulatory sequences. Blood 102: 4487-4492
(2003)
44.
Dzionek, A, Fuchs, A, Schmidt, P, Cremer, S, Zysk, M, Miltenyi, S, Buck,
DW, and Schmitz, J: BDCA-2, BDCA-3, and BDCA-4: three markers for
distinct subsets of dendritic cells in human peripheral blood. J Immunol 165:
6037-6046 (2000)
45.
Dzionek, A, Sohma, Y, Nagafune, J, Cella, M, Colonna, M, Facchetti, F,
Gunther, G, Johnston, I, Lanzavecchia, A, Nagasaka, T, Okada, T, Vermi,
W, Winkels, G, Yamamoto, T, Zysk, M, Yamaguchi, Y, and Schmitz, J:
BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin,
mediates antigen capture and is a potent inhibitor of interferon alpha/beta
induction. J Exp Med 194: 1823-1834 (2001)
46.
Evans, VA, Lal, L, Akkina, R, Solomon, A, Wright, E, Lewin, SR, and
Cameron, PU: Thymic plasmacytoid dendritic cells are susceptible to
productive HIV-1 infection and efficiently transfer R5 HIV-1 to thymocytes in
vitro. Retrovirology 8: 43 (2011)
47.
Fabricius, D, Nussbaum, B, Busch, D, Panitz, V, Mandel, B, Vollmer, A,
Westhoff, MA, Kaltenmeier, C, Lunov, O, Tron, K, Nienhaus, GU,
Jahrsdorfer, B, and Debatin, KM: Antiviral vaccines license T cell responses
by suppressing granzyme B levels in human plasmacytoid dendritic cells. J
Immunol 191: 1144-1153 (2013)
48.
Farkas, L, Beiske, K, Lund-Johansen, F, Brandtzaeg, P, and Jahnsen, FL:
Plasmacytoid dendritic cells (natural interferon- alpha/beta-producing cells)
accumulate in cutaneous lupus erythematosus lesions. Am J Pathol 159:
237-243 (2001)
49.
Finke, JS, Shodell, M, Shah, K, Siegal, FP, and Steinman, RM: Dendritic
cell numbers in the blood of HIV-1 infected patients before and after
changes in antiretroviral therapy. J Clin Immunol 24: 647-652 (2004)
85
50.
Fiola, S, Gosselin, D, Takada, K, and Gosselin, J: TLR9 contributes to the
recognition of EBV by primary monocytes and plasmacytoid dendritic cells.
J Immunol 185: 3620-3631 (2010)
51.
Fiorentini, S, Riboldi, E, Facchetti, F, Avolio, M, Fabbri, M, Tosti, G, Becker,
PD, Guzman, CA, Sozzani, S, and Caruso, A: HIV-1 matrix protein p17
induces human plasmacytoid dendritic cells to acquire a migratory
immature cell phenotype. Proc Natl Acad Sci U S A 105: 3867-3872 (2008)
52.
Fitzgerald-Bocarsly, P and Jacobs, ES: Plasmacytoid dendritic cells in HIV
infection: striking a delicate balance. J Leukoc Biol 87: 609-620 (2010)
53.
Fletcher, JM, Vukmanovic-Stejic, M, Dunne, PJ, Birch, KE, Cook, JE,
Jackson, SE, Salmon, M, Rustin, MH, and Akbar, AN: Cytomegalovirusspecific CD4+ T cells in healthy carriers are continuously driven to
replicative exhaustion. J Immunol 175: 8218-8225 (2005)
54.
Fong, L, Mengozzi, M, Abbey, NW, Herndier, BG, and Engleman, EG:
Productive
infection
of
plasmacytoid
dendritic
cells
with
human
immunodeficiency virus type 1 is triggered by CD40 ligation. J Virol 76:
11033-11041 (2002)
55.
Fonteneau, JF, Larsson, M, Beignon, AS, McKenna, K, Dasilva, I, Amara,
A, Liu, YJ, Lifson, JD, Littman, DR, and Bhardwaj, N: Human
immunodeficiency virus type 1 activates plasmacytoid dendritic cells and
concomitantly induces the bystander maturation of myeloid dendritic cells. J
Virol 78: 5223-5232 (2004)
56.
Freed, EO and Martin, MA: HIVs and Their Replication. In: Knipe, DM and
Howley, OM (Editors) Fields Virology Fifth Edition, Vol. 2, Lippincott
Williams & Wilkins, Philadelphia, p. 2107-2185 (2007)
57.
Froelich, CJ, Zhang, X, Turbov, J, Hudig, D, Winkler, U, and Hanna, WL:
Human granzyme B degrades aggrecan proteoglycan in matrix synthesized
by chondrocytes. J Immunol 151: 7161-7171 (1993)
86
58.
Galluzzi, L, Kepp, O, Morselli, E, Vitale, I, Senovilla, L, Pinti, M, Zitvogel, L,
and Kroemer, G: Viral strategies for the evasion of immunogenic cell death.
J Intern Med 267: 526-542
59.
Gerlini, G, Mariotti, G, Bianchi, B, and Pimpinelli, N: Massive recruitment of
type I interferon producing plasmacytoid dendritic cells in varicella skin
lesions. J Invest Dermatol 126: 507-509 (2006)
60.
Gilliet, M and Liu, YJ: Generation of human CD8 T regulatory cells by CD40
ligand-activated plasmacytoid dendritic cells. J Exp Med 195: 695-704
(2002)
61.
Gondek, DC, Lu, LF, Quezada, SA, Sakaguchi, S, and Noelle, RJ: Cutting
edge: contact-mediated suppression by CD4+CD25+ regulatory cells
involves a granzyme B-dependent, perforin-independent mechanism. J
Immunol 174: 1783-1786 (2005)
62.
Graham, FL, Smiley, J, Russell, WC, and Nairn, R: Characteristics of a
human cell line transformed by DNA from human adenovirus type 5. J Gen
Virol 36: 59-74 (1977)
63.
Grossman, WJ, Verbsky, JW, Tollefsen, BL, Kemper, C, Atkinson, JP, and
Ley, TJ: Differential expression of granzymes A and B in human cytotoxic
lymphocyte subsets and T regulatory cells. Blood 104: 2840-2848 (2004)
64.
Grouard, G, Rissoan, MC, Filgueira, L, Durand, I, Banchereau, J, and Liu,
YJ: The enigmatic plasmacytoid T cells develop into dendritic cells with
interleukin (IL)-3 and CD40-ligand. J Exp Med 185: 1101-1111 (1997)
65.
Guba, SC, Stella, G, Turka, LA, June, CH, Thompson, CB, and Emerson,
SG: Regulation of interleukin 3 gene induction in normal human T cells. J
Clin Invest 84: 1701-1706 (1989)
66.
Guiducci, C, Coffman, RL, and Barrat, FJ: Signalling pathways leading to
IFN-alpha production in human plasmacytoid dendritic cell and the possible
use of agonists or antagonists of TLR7 and TLR9 in clinical indications. J
Intern Med 265: 43-57 (2009)
87
67.
Haas, T, Metzger, J, Schmitz, F, Heit, A, Muller, T, Latz, E, and Wagner, H:
The DNA sugar backbone 2' deoxyribose determines toll-like receptor 9
activation. Immunity 28: 315-323 (2008)
68.
Hadeiba, H and Butcher, EC: Thymus-homing dendritic cells in central
tolerance. Eur J Immunol 43: 1425-1429 (2013)
69.
Hagn, M, Schwesinger, E, Ebel, V, Sontheimer, K, Maier, J, Beyer, T,
Syrovets, T, Laumonnier, Y, Fabricius, D, Simmet, T, and Jahrsdorfer, B:
Human B cells secrete granzyme B when recognizing viral antigens in the
context of the acute phase cytokine IL-21. J Immunol 183: 1838-1845
(2009)
70.
Heibein, JA, Goping, IS, Barry, M, Pinkoski, MJ, Shore, GC, Green, DR,
and Bleackley, RC: Granzyme B-mediated cytochrome c release is
regulated by the Bcl-2 family members bid and Bax. J Exp Med 192: 13911402 (2000)
71.
Heil, F, Ahmad-Nejad, P, Hemmi, H, Hochrein, H, Ampenberger, F, Gellert,
T, Dietrich, H, Lipford, G, Takeda, K, Akira, S, Wagner, H, and Bauer, S:
The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a
strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol 33:
2987-2997 (2003)
72.
Heil, F, Hemmi, H, Hochrein, H, Ampenberger, F, Kirschning, C, Akira, S,
Lipford, G, Wagner, H, and Bauer, S: Species-specific recognition of singlestranded RNA via toll-like receptor 7 and 8. Science 303: 1526-1529 (2004)
73.
Hemmi, H, Takeuchi, O, Kawai, T, Kaisho, T, Sato, S, Sanjo, H,
Matsumoto, M, Hoshino, K, Wagner, H, Takeda, K, and Akira, S: A Toll-like
receptor recognizes bacterial DNA. Nature 408: 740-745 (2000)
74.
Hemmi, H, Kaisho, T, Takeuchi, O, Sato, S, Sanjo, H, Hoshino, K, Horiuchi,
T, Tomizawa, H, Takeda, K, and Akira, S: Small anti-viral compounds
activate immune cells via the TLR7 MyD88-dependent signaling pathway.
Nat Immunol 3: 196-200 (2002)
88
75.
Hendel, A, Hsu, I, and Granville, DJ: Granzyme B releases vascular
endothelial growth factor from extracellular matrix and induces vascular
permeability. Lab Invest 94: 716-725 (2014)
76.
Hochrein, H, O'Keeffe, M, and Wagner, H: Human and mouse plasmacytoid
dendritic cells. Hum Immunol 63: 1103-1110 (2002)
77.
Hodge, S, Hodge, G, Nairn, J, Holmes, M, and Reynolds, PN: Increased
airway granzyme b and perforin in current and ex-smoking COPD subjects.
COPD 3: 179-187 (2006)
78.
Hoeffel, G, Ripoche, AC, Matheoud, D, Nascimbeni, M, Escriou, N, Lebon,
P, Heshmati, F, Guillet, JG, Gannage, M, Caillat-Zucman, S, Casartelli, N,
Schwartz, O, De la Salle, H, Hanau, D, Hosmalin, A, and Maranon, C:
Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity
27: 481-492 (2007)
79.
Hong, HS, Bhatnagar, N, Ballmaier, M, Schubert, U, Henklein, P,
Volgmann, T, Heiken, H, Schmidt, RE, and Meyer-Olson, D: Exogenous
HIV-1 Vpr disrupts IFN-alpha response by plasmacytoid dendritic cells
(pDCs) and subsequent pDC/NK interplay. Immunol Lett 125: 100-104
(2009)
80.
Huang, J, Yang, Y, Al-Mozaini, M, Burke, PS, Beamon, J, Carrington, MF,
Seiss, K, Rychert, J, Rosenberg, ES, Lichterfeld, M, and Yu, XG: Dendritic
cell dysfunction during primary HIV-1 infection. J Infect Dis 204: 1557-1562
(2011)
81.
Huch, JH, Cunningham, AL, Arvin, AM, Nasr, N, Santegoets, SJ,
Slobedman, E, Slobedman, B, and Abendroth, A: Impact of varicella-zoster
virus on dendritic cell subsets in human skin during natural infection. J Virol
84: 4060-4072 (2010)
82.
Hudson, GS, Bankier, AT, Satchwell, SC, and Barrell, BG: The short unique
region of the B95-8 Epstein-Barr virus genome. Virology 147: 81-98 (1985)
89
83.
Iannello, A, Debbeche, O, Martin, E, Attalah, LH, Samarani, S, and Ahmad,
A: Viral strategies for evading antiviral cellular immune responses of the
host. J Leukoc Biol 79: 16-35 (2006)
84.
Ito, T, Kanzler, H, Duramad, O, Cao, W, and Liu, YJ: Specialization,
kinetics, and repertoire of type 1 interferon responses by human
plasmacytoid predendritic cells. Blood 107: 2423-2431 (2006)
85.
Ito, T, Yang, M, Wang, YH, Lande, R, Gregorio, J, Perng, OA, Qin, XF, Liu,
YJ, and Gilliet, M: Plasmacytoid dendritic cells prime IL-10-producing T
regulatory cells by inducible costimulator ligand. J Exp Med 204: 105-115
(2007)
86.
Izaguirre, A, Barnes, BJ, Amrute, S, Yeow, WS, Megjugorac, N, Dai, J,
Feng, D, Chung, E, Pitha, PM, and Fitzgerald-Bocarsly, P: Comparative
analysis of IRF and IFN-alpha expression in human plasmacytoid and
monocyte-derived dendritic cells. J Leukoc Biol 74: 1125-1138 (2003)
87.
Jahrsdorfer, B, Vollmer, A, Blackwell, SE, Maier, J, Sontheimer, K, Beyer,
T, Mandel, B, Lunov, O, Tron, K, Nienhaus, GU, Simmet, T, Debatin, KM,
Weiner, GJ, and Fabricius, D: Granzyme B produced by human
plasmacytoid dendritic cells suppresses T-cell expansion. Blood 115: 11561165 (2010)
88.
Jahrsdörfer, B, Panitz, V, and Fabricius, D: Human Immunodeficiency Virus
Arrests Plasmacytoid Dendritic Cells in a Granzyme Bhigh Tolerogenic
State. J Vaccines Vaccin 7: 337 (2016)
89.
Jarrossay, D, Napolitani, G, Colonna, M, Sallusto, F, and Lanzavecchia, A:
Specialization and complementarity in microbial molecule recognition by
human myeloid and plasmacytoid dendritic cells. Eur J Immunol 31: 33883393 (2001)
90.
Jego, G, Palucka, AK, Blanck, JP, Chalouni, C, Pascual, V, and
Banchereau,
J:
Plasmacytoid
dendritic
cells
induce
plasma
cell
differentiation through type I interferon and interleukin 6. Immunity 19: 225234 (2003)
90
91.
Kadowaki, N, Antonenko, S, Lau, JY, and Liu, YJ: Natural interferon
alpha/beta-producing cells link innate and adaptive immunity. J Exp Med
192: 219-226 (2000)
92.
Kadowaki, N, Ho, S, Antonenko, S, Malefyt, RW, Kastelein, RA, Bazan, F,
and Liu, YJ: Subsets of human dendritic cell precursors express different
toll-like receptors and respond to different microbial antigens. J Exp Med
194: 863-869 (2001)
93.
Kawamura, K, Kadowaki, N, Kitawaki, T, and Uchiyama, T: Virus-stimulated
plasmacytoid dendritic cells induce CD4+ cytotoxic regulatory T cells. Blood
107: 1031-1038 (2006)
94.
Kim, WJ, Kim, H, Suk, K, and Lee, WH: Macrophages express granzyme B
in the lesion areas of atherosclerosis and rheumatoid arthritis. Immunol Lett
111: 57-65 (2007)
95.
Kotenko, SV, Saccani, S, Izotova, LS, Mirochnitchenko, OV, and Pestka, S:
Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10).
Proc Natl Acad Sci U S A 97: 1695-1700 (2000)
96.
Krieg, AM: CpG motifs in bacterial DNA and their immune effects. Annu
Rev Immunol 20: 709-760 (2002)
97.
Kumagai, Y, Kumar, H, Koyama, S, Kawai, T, Takeuchi, O, and Akira, S:
Cutting Edge: TLR-Dependent viral recognition along with type I IFN
positive feedback signaling masks the requirement of viral replication for
IFN-{alpha} production in plasmacytoid dendritic cells. J Immunol 182:
3960-3964 (2009)
98.
Kuritzkes,
DR
and
Walker,
BD:
HIV-1:
Pathogenesis,
Clinical
Manifestations, and Treatment. In: Knipe, DM and Howley, PM (Editors)
Fields Virology Fifth Edition, Vol. 2, Lippincott Williams & Wilkins,
Philadelphia, p. 2187-2214 (2007)
99.
Kvale, EO, Dalgaard, J, Lund-Johansen, F, Rollag, H, Farkas, L, Midtvedt,
K, Jahnsen, FL, Brinchmann, JE, and Olweus, J: CD11c+ dendritic cells
and plasmacytoid DCs are activated by human cytomegalovirus and retain
91
efficient T cell-stimulatory capability upon infection. Blood 107: 2022-2029
(2006)
100.
Kvale, EO, Floisand, Y, Lund-Johansen, F, Rollag, H, Farkas, L, Ghanekar,
S, Brandtzaeg, P, Jahnsen, FL, and Olweus, J: Plasmacytoid DCs regulate
recall responses by rapid induction of IL-10 in memory T cells. Blood 109:
3369-3376 (2007)
101.
Lebedeva, T, Dustin, ML, and Sykulev, Y: ICAM-1 co-stimulates target cells
to facilitate antigen presentation. Curr Opin Immunol 17: 251-258 (2005)
102.
Lee, HK, Lund, JM, Ramanathan, B, Mizushima, N, and Iwasaki, A:
Autophagy-dependent viral recognition by plasmacytoid dendritic cells.
Science 315: 1398-1401 (2007)
103.
Lehmann, C, Harper, JM, Taubert, D, Hartmann, P, Fatkenheuer, G, Jung,
N, van Lunzen, J, Stellbrink, HJ, Gallo, RC, and Romerio, F: Increased
interferon alpha expression in circulating plasmacytoid dendritic cells of
HIV-1-infected patients. J Acquir Immune Defic Syndr 48: 522-530 (2008)
104.
Lehmann, C, Taubert, D, Jung, N, Fatkenheuer, G, van Lunzen, J,
Hartmann, P, and Romerio, F: Preferential upregulation of interferon-alpha
subtype 2 expression in HIV-1 patients. AIDS Res Hum Retroviruses 25:
577-581 (2009)
105.
Lehmann, C, Lafferty, M, Garzino-Demo, A, Jung, N, Hartmann, P,
Fatkenheuer, G, Wolf, JS, van Lunzen, J, and Romerio, F: Plasmacytoid
dendritic cells accumulate and secrete interferon alpha in lymph nodes of
HIV-1 patients. PLoS One 5: e11110 (2010)
106.
Lepelley, A, Louis, S, Sourisseau, M, Law, HK, Pothlichet, J, Schilte, C,
Chaperot, L, Plumas, J, Randall, RE, Si-Tahar, M, Mammano, F, Albert,
ML, and Schwartz, O: Innate sensing of HIV-infected cells. PLoS Pathog 7:
e1001284 (2011)
107.
Lerner, MR, Andrews, NC, Miller, G, and Steitz, JA: Two small RNAs
encoded by Epstein-Barr virus and complexed with protein are precipitated
92
by antibodies from patients with systemic lupus erythematosus. Proc Natl
Acad Sci U S A 78: 805-809 (1981)
108.
Lim, WH, Kireta, S, Russ, GR, and Coates, PT: Human plasmacytoid
dendritic cells regulate immune responses to Epstein-Barr virus (EBV)
infection and delay EBV-related mortality in humanized NOD-SCID mice.
Blood 109: 1043-1050 (2007)
109.
Liu,
YJ:
IPC:
professional
type
1
interferon-producing
cells
and
plasmacytoid dendritic cell precursors. Annu Rev Immunol 23: 275-306
(2005)
110.
Lo, CC, Schwartz, JA, Johnson, DJ, Yu, M, Aidarus, N, Mujib, S, Benko, E,
Hyrcza, M, Kovacs, C, and Ostrowski, MA: HIV delays IFN-alpha production
from human plasmacytoid dendritic cells and is associated with SYK
phosphorylation. PLoS One 7: e37052 (2012)
111.
Loeb, CR, Harris, JL, and Craik, CS: Granzyme B proteolyzes receptors
important to proliferation and survival, tipping the balance toward apoptosis.
J Biol Chem 281: 28326-28335 (2006)
112.
Loenen, WA, Bruggeman, CA, and Wiertz, EJ: Immune evasion by human
cytomegalovirus: lessons in immunology and cell biology. Semin Immunol
13: 41-49 (2001)
113.
Loewendorf, A and Benedict, CA: Modulation of host innate and adaptive
immune defenses by cytomegalovirus: timing is everything. J Intern Med
267: 483-501
114.
Lore, K, Smed-Sorensen, A, Vasudevan, J, Mascola, JR, and Koup, RA:
Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to
antigen-specific CD4+ T cells. J Exp Med 201: 2023-2033 (2005)
115.
Lund, JM, Alexopoulou, L, Sato, A, Karow, M, Adams, NC, Gale, NW,
Iwasaki, A, and Flavell, RA: Recognition of single-stranded RNA viruses by
Toll-like receptor 7. Proc Natl Acad Sci U S A 101: 5598-5603 (2004)
93
116.
Machmach, K, Leal, M, Gras, C, Viciana, P, Genebat, M, Franco, E,
Boufassa,
F, Lambotte, O,
Herbeuval,
JP,
and
Ruiz-Mateos,
E:
Plasmacytoid dendritic cells reduce HIV production in elite controllers. J
Virol 86: 4245-4252 (2012)
117.
Manches, O, Munn, D, Fallahi, A, Lifson, J, Chaperot, L, Plumas, J, and
Bhardwaj, N: HIV-activated human plasmacytoid DCs induce Tregs through
an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest 118:
3431-3439 (2008)
118.
Martinelli, E, Cicala, C, Van Ryk, D, Goode, DJ, Macleod, K, Arthos, J, and
Fauci, AS: HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha}
secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A 104:
3396-3401 (2007)
119.
Martinson, JA, Roman-Gonzalez, A, Tenorio, AR, Montoya, CJ, Gichinga,
CN, Rugeles, MT, Tomai, M, Krieg, AM, Ghanekar, S, Baum, LL, and
Landay, AL: Dendritic cells from HIV-1 infected individuals are less
responsive to toll-like receptor (TLR) ligands. Cell Immunol 250: 75-84
(2007)
120.
Matta, BM, Castellaneta, A, and Thomson, AW: Tolerogenic plasmacytoid
DC. Eur J Immunol 40: 2667-2676 (2010)
121.
Megjugorac, NJ, Young, HA, Amrute, SB, Olshalsky, SL, and FitzgeraldBocarsly, P: Virally stimulated plasmacytoid dendritic cells produce
chemokines and induce migration of T and NK cells. J Leukoc Biol 75: 504514 (2004)
122.
Meier, A, Chang, JJ, Chan, ES, Pollard, RB, Sidhu, HK, Kulkarni, S, Wen,
TF, Lindsay, RJ, Orellana, L, Mildvan, D, Bazner, S, Streeck, H, Alter, G,
Lifson, JD, Carrington, M, Bosch, RJ, Robbins, GK, and Altfeld, M: Sex
differences in the Toll-like receptor-mediated response of plasmacytoid
dendritic cells to HIV-1. Nat Med 15: 955-959 (2009)
94
123.
Metkar, SS, Wang, B, Ebbs, ML, Kim, JH, Lee, YJ, Raja, SM, and Froelich,
CJ: Granzyme B activates procaspase-3 which signals a mitochondrial
amplification loop for maximal apoptosis. J Cell Biol 160: 875-885 (2003)
124.
Meyers, JH, Justement, JS, Hallahan, CW, Blair, ET, Sun, YA, O'Shea, MA,
Roby, G, Kottilil, S, Moir, S, Kovacs, CM, Chun, TW, and Fauci, AS: Impact
of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells.
PLoS One 2: e458 (2007)
125.
Miller, G, Shope, T, Lisco, H, Stitt, D, and Lipman, M: Epstein-Barr virus:
transformation, cytopathic changes, and viral antigens in squirrel monkey
and marmoset leukocytes. Proc Natl Acad Sci U S A 69: 383-387 (1972)
126.
Miller, G and Lipman, M: Release of infectious Epstein-Barr virus by
transformed marmoset leukocytes. Proc Natl Acad Sci U S A 70: 190-194
(1973)
127.
Mocarski Jr., ES, Shenk, T, and Pass, RF: Cytomegaloviruses. In: Knipe,
DM and Howley, PM (Editors) Fields Virology Fifth Edition, Vol. 2,
Lippincott, Williams & Wilkins, Philadelphia, p. 2701-2772 (2007)
128.
Moseman, EA, Liang, X, Dawson, AJ, Panoskaltsis-Mortari, A, Krieg, AM,
Liu, YJ, Blazar, BR, and Chen, W: Human plasmacytoid dendritic cells
activated by CpG oligodeoxynucleotides induce the generation of
CD4+CD25+ regulatory T cells. J Immunol 173: 4433-4442 (2004)
129.
Munz, C: Dendritic cells during Epstein Barr virus infection. Front Microbiol
5: 308 (2014)
130.
Nakano, H, Yanagita, M, and Gunn, MD: CD11c(+)B220(+)Gr-1(+) cells in
mouse lymph nodes and spleen display characteristics of plasmacytoid
dendritic cells. J Exp Med 194: 1171-1178 (2001)
131.
Naniche,
D:
Generalized
immunosuppression:
individual
viruses,
intertwined targets. Virology 275: 227-232 (2000)
132.
Nascimbeni, M, Perie, L, Chorro, L, Diocou, S, Kreitmann, L, Louis, S,
Garderet, L, Fabiani, B, Berger, A, Schmitz, J, Marie, JP, Molina, TJ,
95
Pacanowski, J, Viard, JP, Oksenhendler, E, Beq, S, Abehsira-Amar, O,
Cheynier, R, and Hosmalin, A: Plasmacytoid dendritic cells accumulate in
spleens from chronically HIV-infected patients but barely participate in
interferon-alpha expression. Blood 113: 6112-6119 (2009)
133.
Nemerow, GR and Cooper, NR: Isolation of Epstein Barr-virus and studies
of its neutralization by human IgG and complement. J Immunol 127: 272278 (1981)
134.
Nestle, FO, Conrad, C, Tun-Kyi, A, Homey, B, Gombert, M, Boyman, O,
Burg, G, Liu, YJ, and Gilliet, M: Plasmacytoid predendritic cells initiate
psoriasis through interferon-alpha production. J Exp Med 202: 135-143
(2005)
135.
Nussbaum, BL: Effects of commonly used antiviral vaccines on human
plasmacytoid dendritic cells. Med Dissertation, Ulm University (2013)
136.
O'Brien, M, Manches, O, Sabado, RL, Baranda, SJ, Wang, Y, Marie, I,
Rolnitzky, L, Markowitz, M, Margolis, DM, Levy, D, and Bhardwaj, N:
Spatiotemporal trafficking of HIV in human plasmacytoid dendritic cells
defines
a
persistently
IFN-alpha-producing
and
partially
matured
phenotype. J Clin Invest 121: 1088-1101 (2011)
137.
O'Keeffe, M, Hochrein, H, Vremec, D, Caminschi, I, Miller, JL, Anders, EM,
Wu, L, Lahoud, MH, Henri, S, Scott, B, Hertzog, P, Tatarczuch, L, and
Shortman, K: Mouse plasmacytoid cells: long-lived cells, heterogeneous in
surface phenotype and function, that differentiate into CD8(+) dendritic cells
only after microbial stimulus. J Exp Med 196: 1307-1319 (2002)
138.
Pacanowski, J, Kahi, S, Baillet, M, Lebon, P, Deveau, C, Goujard, C,
Meyer, L, Oksenhendler, E, Sinet, M, and Hosmalin, A: Reduced blood
CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary
HIV-1 infection. Blood 98: 3016-3021 (2001)
139.
Patterson, S, Rae, A, Hockey, N, Gilmour, J, and Gotch, F: Plasmacytoid
dendritic cells are highly susceptible to human immunodeficiency virus type
1 infection and release infectious virus. J Virol 75: 6710-6713 (2001)
96
140.
Penna, G, Sozzani, S, and Adorini, L: Cutting edge: selective usage of
chemokine receptors by plasmacytoid dendritic cells. J Immunol 167: 18621866 (2001)
141.
Pichlmair, A and Reis e Sousa, C: Innate recognition of viruses. Immunity
27: 370-383 (2007)
142.
Pitabut, N, Sakurada, S, Tanaka, T, Ridruechai, C, Tanuma, J, Aoki, T,
Kantipong, P, Piyaworawong, S, Kobayashi, N, Dhepakson, P, Yanai, H,
Yamada, N, Oka, S, Okada, M, Khusmith, S, and Keicho, N: Potential
function of granulysin, other related effector molecules and lymphocyte
subsets in patients with TB and HIV/TB coinfection. Int J Med Sci 10: 10031014 (2013)
143.
Plaut, M, Pierce, JH, Watson, CJ, Hanley-Hyde, J, Nordan, RP, and Paul,
WE: Mast cell lines produce lymphokines in response to cross-linkage of Fc
epsilon RI or to calcium ionophores. Nature 339: 64-67 (1989)
144.
Quan, TE, Roman, RM, Rudenga, BJ, Holers, VM, and Craft, JE: EpsteinBarr virus promotes interferon-alpha production by plasmacytoid dendritic
cells. Arthritis Rheum 62: 1693-1701 (2010)
145.
Reitano, KN, Kottilil, S, Gille, CM, Zhang, X, Yan, M, O'Shea, MA, Roby, G,
Hallahan, CW, Yang, J, Lempicki, RA, Arthos, J, and Fauci, AS: Defective
plasmacytoid dendritic cell-NK cell cross-talk in HIV infection. AIDS Res
Hum Retroviruses 25: 1029-1037 (2009)
146.
Rickinson, AB and Kieff, E: Epstein-Barr Virus. In: Knipe, DM and Howley,
PM (Editors) Fields Virology Fifth Edition, Vol. 2, Lippincott, Williams &
Wilkins, Philadelphia, p. 2655-2700 (2007)
147.
Rissoan, MC, Duhen, T, Bridon, JM, Bendriss-Vermare, N, Peronne, C, de
Saint Vis, B, Briere, F, and Bates, EE: Subtractive hybridization reveals the
expression of immunoglobulin-like transcript 7, Eph-B1, granzyme B, and 3
novel transcripts in human plasmacytoid dendritic cells. Blood 100: 32953303 (2002)
97
148.
Romero, V and Andrade, F: Non-apoptotic functions of granzymes. Tissue
Antigens 71: 409-416 (2008)
149.
Ronnblom, L and Pascual, V: The innate immune system in SLE: type I
interferons and dendritic cells. Lupus 17: 394-399 (2008)
150.
Russell, JH and Ley, TJ: Lymphocyte-mediated cytotoxicity. Annu Rev
Immunol 20: 323-370 (2002)
151.
Sabado, RL, O'Brien, M, Subedi, A, Qin, L, Hu, N, Taylor, E, Dibben, O,
Stacey, A, Fellay, J, Shianna, KV, Siegal, F, Shodell, M, Shah, K, Larsson,
M, Lifson, J, Nadas, A, Marmor, M, Hutt, R, Margolis, D, Garmon, D,
Markowitz, M, Valentine, F, Borrow, P, and Bhardwaj, N: Evidence of
dysregulation of dendritic cells in primary HIV infection. Blood 116: 38393852 (2010)
152.
Santini, SM, Di Pucchio, T, Lapenta, C, Parlato, S, Logozzi, M, and
Belardelli, F: The natural alliance between type I interferon and dendritic
cells and its role in linking innate and adaptive immunity. J Interferon
Cytokine Res 22: 1071-1080 (2002)
153.
Schmidt, B, Ashlock, BM, Foster, H, Fujimura, SH, and Levy, JA: HIVinfected cells are major inducers of plasmacytoid dendritic cell interferon
production, maturation, and migration. Virology 343: 256-266 (2005)
154.
Schneider, K, Meyer-Koenig, U, and Hufert, FT: Human cytomegalovirus
impairs the function of plasmacytoid dendritic cells in lymphoid organs.
PLoS One 3: e3482 (2008)
155.
Severa, M, Giacomini, E, Gafa, V, Anastasiadou, E, Rizzo, F, Corazzari, M,
Romagnoli, A, Trivedi, P, Fimia, GM, and Coccia, EM: EBV stimulates TLRand
autophagy-dependent
pathways
and
impairs
maturation
in
plasmacytoid dendritic cells: implications for viral immune escape. Eur J
Immunol 43: 147-158 (2013)
156.
Shi, L, Kam, CM, Powers, JC, Aebersold, R, and Greenberg, AH:
Purification of three cytotoxic lymphocyte granule serine proteases that
98
induce apoptosis through distinct substrate and target cell interactions. J
Exp Med 176: 1521-1529 (1992)
157.
Shigematsu, H, Reizis, B, Iwasaki, H, Mizuno, S, Hu, D, Traver, D, Leder,
P, Sakaguchi, N, and Akashi, K: Plasmacytoid dendritic cells activate
lymphoid-specific genetic programs irrespective of their cellular origin.
Immunity 21: 43-53 (2004)
158.
Shortman, K and Liu, YJ: Mouse and human dendritic cell subtypes. Nat
Rev Immunol 2: 151-161 (2002)
159.
Siegal, FP, Kadowaki, N, Shodell, M, Fitzgerald-Bocarsly, PA, Shah, K, Ho,
S, Antonenko, S, and Liu, YJ: The nature of the principal type 1 interferonproducing cells in human blood. Science 284: 1835-1837 (1999)
160.
Soumelis, V, Scott, I, Gheyas, F, Bouhour, D, Cozon, G, Cotte, L, Huang, L,
Levy, JA, and Liu, YJ: Depletion of circulating natural type 1 interferonproducing cells in HIV-infected AIDS patients. Blood 98: 906-912 (2001)
161.
Spaeny-Dekking, EH, Hanna, WL, Wolbink, AM, Wever, PC, Kummer, JA,
Swaak, AJ, Middeldorp, JM, Huisman, HG, Froelich, CJ, and Hack, CE:
Extracellular granzymes A and B in humans: detection of native species
during CTL responses in vitro and in vivo. J Immunol 160: 3610-3616
(1998)
162.
Steinman, RM and Cohn, ZA: Identification of a novel cell type in peripheral
lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J
Exp Med 137: 1142-1162 (1973)
163.
Strik, MC, de Koning, PJ, Kleijmeer, MJ, Bladergroen, BA, Wolbink, AM,
Griffith, JM, Wouters, D, Fukuoka, Y, Schwartz, LB, Hack, CE, van Ham,
SM, and Kummer, JA: Human mast cells produce and release the cytotoxic
lymphocyte associated protease granzyme B upon activation. Mol Immunol
44: 3462-3472 (2007)
164.
Swiecki, M and Colonna, M: Unraveling the functions of plasmacytoid
dendritic cells during viral infections, autoimmunity, and tolerance. Immunol
Rev 234: 142-162 (2010)
99
165.
Tak, PP, Spaeny-Dekking, L, Kraan, MC, Breedveld, FC, Froelich, CJ, and
Hack, CE: The levels of soluble granzyme A and B are elevated in plasma
and synovial fluid of patients with rheumatoid arthritis (RA). Clin Exp
Immunol 116: 366-370 (1999)
166.
ten Berge, IJ, Wever, PC, Wolbink, AM, Surachno, J, Wertheim, PM,
Spaeny, LH, and Hack, CE: Increased systemic levels of soluble
granzymes A and B during primary cytomegalovirus infection after renal
transplantation. Transplant Proc 30: 3972-3974 (1998)
167.
Tillieux, SL, Halsey, WS, Thomas, ES, Voycik, JJ, Sathe, GM, and
Vassilev, V: Complete DNA sequences of two oka strain varicella-zoster
virus genomes. J Virol 82: 11023-11044 (2008)
168.
Trapani,
JA
and
Smyth,
MJ:
Functional
significance
of
the
perforin/granzyme cell death pathway. Nat Rev Immunol 2: 735-747 (2002)
169.
Treilleux, I, Blay, JY, Bendriss-Vermare, N, Ray-Coquard, I, Bachelot, T,
Guastalla, JP, Bremond, A, Goddard, S, Pin, JJ, Barthelemy-Dubois, C, and
Lebecque, S: Dendritic cell infiltration and prognosis of early stage breast
cancer. Clin Cancer Res 10: 7466-7474 (2004)
170.
Tschopp, CM, Spiegl, N, Didichenko, S, Lutmann, W, Julius, P, Virchow,
JC, Hack, CE, and Dahinden, CA: Granzyme B, a novel mediator of allergic
inflammation: its induction and release in blood basophils and human
asthma. Blood 108: 2290-2299 (2006)
171.
Varani, S, Cederarv, M, Feld, S, Tammik, C, Frascaroli, G, Landini, MP,
and Soderberg-Naucler, C: Human cytomegalovirus differentially controls B
cell and T cell responses through effects on plasmacytoid dendritic cells. J
Immunol 179: 7767-7776 (2007)
172.
Varani, S, Rossini, G, Mastroianni, A, Tammik, C, Frascaroli, G, Landini,
MP, Castellani, G, and Soderberg-Naucler, C: High TNF-alpha and IL-8
levels predict low blood dendritic cell counts in primary cytomegalovirus
infection. J Clin Virol 53: 360-363 (2012)
100
173.
Vermi, W, Bonecchi, R, Facchetti, F, Bianchi, D, Sozzani, S, Festa, S,
Berenzi, A, Cella, M, and Colonna, M: Recruitment of immature
plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid
dendritic cells in primary cutaneous melanomas. J Pathol. 200: 255-268.
(2003)
174.
Vieira, P, de Waal-Malefyt, R, Dang, MN, Johnson, KE, Kastelein, R,
Fiorentino, DF, deVries, JE, Roncarolo, MG, Mosmann, TR, and Moore,
KW: Isolation and expression of human cytokine synthesis inhibitory factor
cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI.
Proc Natl Acad Sci U S A 88: 1172-1176 (1991)
175.
Villadangos, JA and Young, L: Antigen-presentation properties of
plasmacytoid dendritic cells. Immunity 29: 352-361 (2008)
176.
Wei, S, Kryczek, I, Zou, L, Daniel, B, Cheng, P, Mottram, P, Curiel, T,
Lange, A, and Zou, W: Plasmacytoid dendritic cells induce CD8+ regulatory
T cells in human ovarian carcinoma. Cancer Res. 65: 5020-5026. (2005)
177.
Wieckowski, E, Wang, GQ, Gastman, BR, Goldstein, LA, and Rabinowich,
H: Granzyme B-mediated degradation of T-cell receptor zeta chain. Cancer
Res 62: 4884-4889 (2002)
178.
Wu, L and Liu, YJ: Development of dendritic-cell lineages. Immunity 26:
741-750 (2007)
179.
Yu, HR, Huang, HC, Kuo, HC, Sheen, JM, Ou, CY, Hsu, TY, and Yang, KD:
IFN-alpha production by human mononuclear cells infected with varicellazoster virus through TLR9-dependent and -independent pathways. Cell Mol
Immunol 8: 181-188 (2011)
180.
Zhang, Z, Fu, J, Zhao, Q, He, Y, Jin, L, Zhang, H, Yao, J, Zhang, L, and
Wang, FS: Differential restoration of myeloid and plasmacytoid dendritic
cells in HIV-1-infected children after treatment with highly active
antiretroviral therapy. J Immunol 176: 5644-5651 (2006)
101
181.
Zhou, D, Kang, KH, and Spector, SA: Production of interferon alpha by
human immunodeficiency virus type 1 in human plasmacytoid dendritic cells
is dependent on induction of autophagy. J Infect Dis 205: 1258-1267 (2012)
182.
Zou, W, Machelon, V, Coulomb-L'Hermin, A, Borvak, J, Nome, F, Isaeva, T,
Wei, S, Krzysiek, R, Durand-Gasselin, I, Gordon, A, Pustilnik, T, Curiel, DT,
Galanaud, P, Capron, F, Emilie, D, and Curiel, TJ: Stromal-derived factor-1
in human tumors recruits and alters the function of plasmacytoid precursor
dendritic cells. Nat Med 7: 1339-1346 (2001)
102