* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download the immune response
Lymphopoiesis wikipedia , lookup
DNA vaccination wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Autoimmunity wikipedia , lookup
Complement system wikipedia , lookup
Immune system wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Adaptive immune system wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Innate immune system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
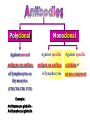
Polyclonal B cell response wikipedia , lookup
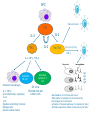
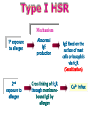
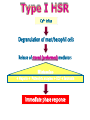
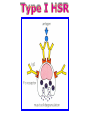
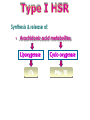
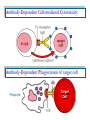
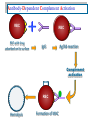
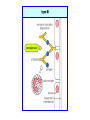
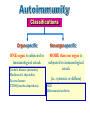
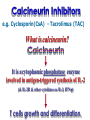
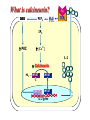
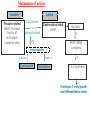
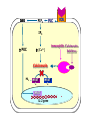
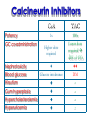
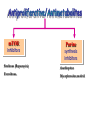
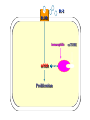
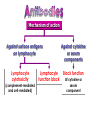
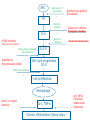
Immuno-Pharmacology Dr. Dalia El Tanbouly • Immunopharmacology: study of drugs that modulate immune response (↑ or ↓). • Immune system consists of: – Organs:1ry(Thymus, bone marrow) 2ry (spleen, lymph nodes) – Cell types: neutrophils, monocytes, natural killer cells, etc. – Molecules: complement component. • Communication among elements: – Surface receptors – Soluble molecules (cytokines). Targets of Immune Response: – Invading organisms. – Growing neoplastic cells. • Immune system must distinguish self from non-self. • Foreign substances that elicit a specific immune response are called antigens. Types of Immune Response: Innate (natural): • 1st line defense • Non-specific. Adaptive (acquired): • Specific. • Have memory. • Subdivided into: – Humoral (B-lymphocytes). – Cellular (T-lymphocytes). Characteristics of Innate & Acquired Immune Responses Innate: Acquired: • Onset: Immediate. • Onset: Days to weeks. • Does not require priming. • Require priming. • Effectors: • Effectors: Physical: skin and mucous membranes. Cellular: macrophages, neutrophils, mast cells, natural killer cells, etc. Biochemical: cytokines, lysozymes and complement (cell lysis (MAC), opsonization C3b, chemotaxis C5a) Cellular: – B-lymphocytes. – T-lymphocytes. • T helpers (CD4). • T cytotoxic (CD8). – Phagocytes (APC). Biochemical: cytokines, lysozymes, complement and immunoglobulins • Onset upon re-exposure: • Onset upon re-exposure: the same. quicker. • Memory: • Memory Present Absent Cytokines • Soluble small peptides used by the immune system to communicate & influence cellular functions. • Involved in innate & adaptive immunity. • Chemokines: low molecular weight cytokines act as chemoattractants e.g Interleukin-8 (IL-8), which induces neutrophils to leave the bloodstream and enter into the surrounding tissue Monocyte chemoattractant protein-1 (MCP-1) which induces monocytes to leave the bloodstream and enter the surrounding tissue to become tissue macrophages. APC Memory B-cells TH IL-2 IL-2 IL-2 TH1 TH1/TH2 IL-2, IL-4, IL-5, INF-γ IL-2, INF-γ, TNF-β Activated NK cell Activated macrophages IL-1, TNF-α (pro inflammatory cytokines) IL-12 IL-8 Ingestion and killing microbes Damage cells Remove cellular debris Activated Cytotoxic T cell Kill virus infected cells ant tumor cells IgE IgA IgG IgM -Neutralization of microbes and toxins -Opsonization of antigens for phacocytosis by macrophages and neutrophils. -Activation of classical pathway of complement (lysis) -Antibody-dependent cellular cytotoxicity by NK cells. Examples of some cytokines: • IL-1 & TNF-α (pro inflammatory cytokines) increase vascular permeability promoting inflammation. • IL-2 (T cell growth factor): T- cell proliferation and differentiation into effector and memory cells. • IL-4 (B cell growth factor): TH2 derived growth factor, essential for IgE production • IFNs (α, β ): Possess antiviral effects. • IFNγ: Macrophage activation, essential for IgG production, possess antiviral effects. • IL-12: Produced differentiation. by macrophage promoting TH1 Abnormal Immune Response: • Abnormal impaired immune response → Immunodeficiency. • Abnormal exaggerated immune response: Hypersensitivity reactions. Autoimmune diseases. Antibody - mediated Type I: Atopy, Allergy, Anaphylactic HS, immediate Type II: Cytotoxic Type III: Immune complex Cell - mediated Type VI: DTH (Atopy - Allergy – Anaphylactic - Immediate) Mechanism 1st exposure to allergen 2nd exposure to allergen Abnormal IgE production Cross linking of fcR through membranebound IgE by allergen IgE fixed on the surface of mast cells or basophils via fcR (Sensitization) Ca2+ influx Ca2+ influx Degranulation of mast/basophil cells Release of stored (preformed) mediators Histamine + Heparin + Proteolytic enzymes + ECF + Serotonin Immediate phase response Synthesis & release of: • Arachidonic acid metabolites Lipoxygenase LTs Cyclo-oxygenase PGs - TX Immediate Phase Histamine LTC4 / LTD4 Vasodilatation, Vascular permeability, transient contraction of smooth muscle Prolonged smooth muscle contraction Bronchoconstriction - Bronchial secretions Mucosal edema Histamine – Kinins Vasodilatation - PG Vascular permeability edema Proteasese Tissue damage – inflammation Cytokines ( TNFαIL-4), LTB4 Attraction of leucocytes (eosinophils & neutrophils ) The attracted eosinophils & neutrophils release proteases & mediators Late phase response Clinical Manifestations LOCAL At the sites in which mast cells accumulate Main organ affected Skin (contact) Nose & Eyes (contact) Disease Eczema - Urticaria (hives) Rhinitis, Conjunctivitis (hay fever) Lung Allergic bronchial (inhalation) asthma GIT Allergic (ingestioin) gastroenteropathy SYSTEMIC Anaphylaxis Due to administration of (as by injection) Release of Severe life-threatening hypotension – airway obstruction due to laryngeal edema (Ab mediated cytotoxicity) Mechanisms Generated by actions of antibodies usually IgG or IgM against an epitope on host cell membrane or extracellular matrix . which may be non self molecules Drug or microbial toxin (hapten) is passively adsorbed onto cell membrane Changes these self structures to be antigenic Production of antibody (IgG or IgM) that is directed against it OR a self molecules Antibody-mediated AID (Cytotoxic) Mechanisms The bound antibody stimulates the cell damage by a number of effector mechanisms Antibody-Dependent Cell-mediated Cytotoxicity Antibody-Dependent Phagocytosis of target cell Antibody-Dependent Complement Activation Antibody-Dependent disruption of cellular function: e.g.: Antibodies against cell surface receptor, which may be blocking or stimulating antibodies Antibody-Dependent Cell-mediated Cytotoxicity Antibody-Dependent Phagocytosis of target cell Target Cell Phagocyte FCR Antibody-Dependent Complement Activation RBC RBC with drug adsorbed on its surface RBC IgG Ag/Ab reaction Complement activation RBC Hemolysis Formation of MAC C (Cytotoxic) Examples of Antibody-mediated AID 1. Myasthenia gravis (autoantibodies against Ach receptors in MEP). 2. Hashimoto’s thyroiditis (autoantibodies against thyroid cells). (Cytotoxic) Examples of Drug-induced T2HSR Examples: • Penicillin – phenacetin – quinidine adsorbed on RBC surface hemolysis Hemolytic anemia • Quinine adsorbed on platelet surface platelet lysis thrombocytopenia (Cytotoxic) Example of Microbial induced T2HSR Salmonella lipopolysaccaride endotoxin adsorbed on RBC hemolysis. (Hapten) Streptococcus is rich in an antigen IgG and IgM generated against protein can cross-react with cardiac of the heart impair cardiac reactivity) called M protein. streptococcus M tissues and valves functions (cross (Immune Complex) Mechanism Production of antibody ( IgM, IgG) that is directed against circulating in or in Self molecule Antibodymediated AID Non-self molecule (antigen-antibody complexes) that circulate in the blood high pressure vessels (e.g. renal glomeruli – synovium). release of mediators, ROS, lysosomal enzymes Inflammation (VASCULITIS) Damage to the vessel wall + VD & increased vascular permeability + ( microthrombi) release histamine vasodilation, increased Vascular permeability (VASCULITIS) (Immune Complex) For antigen that is circulating in the blood: Exogenous Post Streptococcal Glomerulonephritis Streptococcal cell wall antigens immune comlplex Nephritis Endogenous (AID) Rheumatiod arthritis caused by deposition of immune complexes in joints. The IgG in the immune complexes can become an antigen, stimulating the production of IgM against the bound IgG. The anti-IgG IgM is also termed the rheumatoid factor extensive damage to bone and cartilage and joint dysfunction. cartilage and joint dysfunction Systemic lupus erythematosus (SLE) Arises from autoantibodies formed against fragments of single or double stranded DNA and some chromosomal proteins (e.g. histones). Because these molecules are widespread throughout the body, the inflammation is broadly distributed Nephritis Nephritis, Skin lesions and Arthritis (Delayed) Mechanism 1. CD4+ (Th-1)-mediated: 1. CD4+ (Th-1) cells interact with processed presented antigen Release of cytokines (e.g. IFN - TNFβ) Activation of macrophages 2. Activated macrophages release: • Lytic enzymes, inflammatory cytokines (e.g. TNFα, IL-1) Inflammatory response & Tissue injury. • IL-12 Stimulates Th-1 to release more IFN - TNFβ Continual cycle Chronic exposure to the antigen Excessive accumulation & activation of macrophages Giant cells Epithelioid cells Granuloma formation (The attempt of the body to isolate a site of persistent stimulus) (Delayed) Mechanism 2. CD8+ (CTL)-mediated: CD8+ cells interact with processed presented antigen Release of killing enzymes cytolysis & inflammatory responses. Examples • Chronic infectious diseases: (bacterial – viral – protozoal - fungal). • Leprosy Contact dermatitis: (haptens + skin proteins immunogen). • • Graft rejection. AID: Multiple sclerosis (Myelin basic protein) and Crohn's disease Graft rejection Contact dermatitis When the body produces immune response against itself (i.e. loss of self-tolerance) Mechanisms 1. Molecular mimicry 2. Activation of anergized auto-reactive T-cells 3. Loss of suppression of auto-reactive T-cells 4. Alteration of normal proteins 5. Release of sequestered antigens 1. Molecular mimicry Some pathogens (bacteria or virus) have epitopes that close similar to normal protein in host tissue Cross-reactivity Infection is frequently associated with development of autoimmunity…Why? 1. Molecular mimicry Rheumatic fever following Streptococcus pyogenes infection Molecular mimicry between M protein of S. pyogenes & the myosin of cardiac muscle & to some degree with molecules on joints & kidneys. Antibodies against M proteins cross-react with myosin in myocardium & joint tissue Rheumatic fever. 2. Activation of anergized auto-reactive T-cell Macrophages that are activated by infection generate elevated levels of cytokines that may activate anergized auto-reactive T-cell Infection is frequently associated with development of autoimmunity…Why? 3. Loss of suppression of auto-reactive T cells Tolerance to self protein can be induced by regulatory (suppressor) cells which diminish the activity of possible auto-reactive T cells. Decrease in no. of regulatory cells (as happen with age) Increases the risk of activation of auto-reactive T cells Autoimmunity. 4. Alteration of normal proteins Neoantigens Self Proteins OR Formation of neoantigen to immune system elicit immune responses Hapten Small molecule that stimulates the production of antibody molecules only when conjugated to a larger molecule, called a carrier molecule. e.g. drug-induced hemolytic anemia. Drugs capable of causing hemolytic anemia include: penicillin, cephalosporins, sulfonamide, quinine 5. Release of sequestered antigens Some self-molecules are normally sequestered (hidden) from immune system by specialized anatomic structure: • Certain tissues (sperm, lens). Damage by Infection-Chemical-Radiation…. Release or exposure of the hidden self-molecules to immune system & elicit immune responses. Classifications Organ-specific ONE organ is subjected to immunological attack Crohn’s disease (intestine) Hashimoto’s thyroiditis Graves disease T1DM (insulin-dependant) Non-organ-specific MORE than one organ is subjected to immunological attack (i.e. systemic or diffuse) SLE Rheumatoid arthritis Classifications Humoral-associated autoimmune disease Pernicious anemia Myasthenia gravis Hashimoto’s thyroiditis Graves disease Systemic lupus erythematosus N.B Cell mediatedautoimmune disease Insulin dependent diabetes mellitus Crohn’s disease Multiple sclerosis Rheumatiod arthritis provides an example of autoimmune disease that involves both humoral and cellmediated injury Immunosuppressive Drugs Therapeutic Uses Used to the immune response in Autoimmune diseases Transplantation Immunosuppressive Drugs Common Adverse Effects Nonspecifically suppress the entire immune system risks of Infections Cancers Immunosuppressive Drugs Drug Classes 1. Glucocorticoids 2. Calcineurin inhibitors 3. Antiproliferative/antimetabolites 4. Antibodies/Fusion proteins e.g. Cyclosporin(CsA) - Tacrolimus (TAC) What is calcineurin? It is a cytoplasmic phosphatase enzyme involved in antigen-triggered synthesis of IL-2 (& IL-2R & other cytokines as IL-3, IFN-) T cells growth and differentiation. What is calcineurin? DAG PLC PIP2 Ag TCR IP3 PKC [Ca2+] IL-2 Calcineurin PO4 NFAT c NFAT n NFAT c NFAT c IL-2 gene Mechanism of action inactive active Phosphorylated NFAT (Nuclear Factor of Activated T Lymphocytes) Calcineurin (phosphatase) Dephosphorylated NFAT (-) Cyclosporin (+) NFAT-GENE complex immunophilins cyclophilin Nucleus FKBP-12 Tacrolimus (+) ↑ IL-2 synthesis Prototypic T-cells growth and differentiation factor DAG PLC PIP2 TCR IP3 PKC [Ca2+] Calcineurin PO4 NFAT c NFAT c NFAT n IL-2 gene Immunophillin Calcineurin Inhibitor Cyclosporine - Tacrolimus Adverse Effects Nephrotoxicity (major) Hepatotoxicity Renal & Liver functions should be periodically monitored Neurotoxicity (tremor, hallucinations, seizures). Hyperglycemia & diabetes Cyclosporine - Tacrolimus Adverse Effects Hypertension Hyperkalemia avoid use of K-sparing diuretics. Anaphylactoid reactions Hirsutism Gum hyperplasia Hypercholesterolemia Hyperuricemia CsA TAC 1x 100x Higher dose required Lower dose required SE of GCs Nephrotoxicity + ++ Blood glucose Glucose intolerance DM Hirsutism + - Gum hyperplasia + - Hypercholesterolemia + - Hyperuricemia + - Potency GC co-administration Cyclosporine - Tacrolimus Drug Interactions Co-administration of NSAIDs and any drug that causes nephrotoxicity nephrotoxicity Cyclosporine + tacrolimus nephrotoxicity (Wait for at least 24h if switching from cyclosporine to tacrolimus). Calcineurin inhibitors especially tacrolimus + glucocorticoids risk of diabetes. mTOR inhibitors Purine synthesis inhibitors Sirolimus (Rapamycin) Azathioprine Everolimus Mycophenolate mofetil What is mTOR? mTOR: mammalian Target Of Rapamycin A key protein kinase enzyme responsible for cell-cycle progression Cell proliferation IL-2 IL-2R Immunophillin mTOR Proliferation mTORI Sirolimus - Everolimus Mechanism of action S Sirolimus immunophilins FKBP-12 (-) mTOR G 1 Blocks cell-cycle progression induced by IL-2 & other T-cell growth factors. (Inhibits the cellular response to IL-2) Sirolimus - Everolimus Adverse Effects Hypercholesterolemia (may require ttt) Myelosuppression anemia, leukopenia, thrombocytopenia Fever, delayed wound healing, & GIT effects. An additional adverse effect noted with everolimus is angioedema Sirolimus - Everolimus Drug Interactions Cyclosporine + sirolimus Sirolimus cyclosporine-induced nephrotoxicity. Cyclosporine sirolimus-induced hyperlipidemia myelosuppression. (Administration of two drugs should be separated by time). & Azathioprine Mechanism of action Azathioprine 6-mercaptopurine de novo purine synthesis ↓ DNA synthesis (S-phase) lymphocyte proliferation. Azathioprine Adverse Effects Myelosuppression leukopenia (common), thrombocytopenia (less common), &/or anemia (uncommon). Hepatotoxicity (mild) Alopecia, skin eruptions GIT toxicity (N,V) Pancreatitis Azathioprine Drug Interactions Azathioprine 6-MP Xan Ox 6-thiouric acid urine + Allopurinol xanthine oxidase inhibitor level of azathioprine …SO….. azathioprine dose must be decreased or avoid these combination). + Myelosuppressive drugs risk of myelosuppression Mycophenolate mofetil Mechanism of action Mycophenolate mofetil (Prodrug) Mycophenolic acid (MPA) (Active drug) Selective, non-competitive, reversible inhibition for inosine monophosphate dehydrogenase de novo guanine synthesis lymphocyte proliferation & function Mycophenolate mofetil Mechanism of action B & T lymphocytes are highly dependent on de novo purine biosynthesis pathway for cell proliferation, while other cell types can generate purines through other pathways MPA lymphocyte proliferation & functions Adverse Effects: GIT effects, leukopenia and anemia Genetically engineered protein molecules 1. Antibodies 2. Fusion proteins Polyclonal Against several antigens on surface of lymphocytes or thymocytes (CD3,CD4,CD8, TCR) Example: Antithymocyte globulin Antilymphocyte globulin Monoclonal Against specific antigen on surface of lymphocytes Against specific cytokine or serum component Nomenclature Animal Chimeric Humanized Human …….omab …….ximab …….zumab …….umab …….amab …….emab More antigenic less antigenic Mechanism of action Against surface antigens on lymphocyte Lymphocyte cytotoxicity (complement-mediated and cell-mediated) Lymphocyte function block Against cytokine or serum components Block function of cytokine or serum component Polyclonal 1. Antithymocyte globulin: • Source: IgG from serum of rabbits immunized with human thymocytes. • Mechanism: direct cytotoxicity to circulating lymphocytes ➙ by direct cytotoxicity (both complement and cellmediated) and block lymphocyte function Adverse effects: Fever, chills, headache, tremor, NVD, generalized weakness & pain, skin reactions, cardiorespiratory, CNS disorders. To minimize this: a. Premedication è GCs, acetaminophen and antihistaminics to avoid allergy. b. Administration by slow infusion (over 4 to 6 hours) into large diameter vessel. Anaphylaxis reaction Serum sickness Hematological complications (leukopenia and thrombocytopenia). Monoclonal Against antigen on surface of lymphocytes 1. Muromonab-CD3: Source: mouse monoclonal antibodies. • Mechanism: Binding to the CD3 protein results in a disruption of T-lymphocyte function, because access of antigen to the recognition site is blocked. • Depletion of lymphocytes due to direct cytotoxicity. Adverse Effects may follow the first dose as Initial binding of muromonab-CD3 to the antigen transiently activates the T cell cytokine release (cytokine storm ) Toxicity • The symptoms can range from a mild, flu-like illness to a lifethreatening, shock-like reaction. • The symptoms (30 min after infusion) frequency & severity decrease with subsequent doses. • To minimize such reactions: Pre-medication with corticosteroids, acetaminophen, &/or an antihistaminic. Administration by slow infusion (over 4 to 6 hours) into large diameter vessel. 2. IL-2 receptor antagonist (Daclizumab and Basiliximab). • Binds to IL-2 receptors → ↓ IL-2-induced T lymphocytes activation. • Basiliximab is about 10-fold more potent than daclizumab 3. IL-1 receptor antagonist (Anakinra) • it binds to the IL-1 receptors → preventing actions of IL-1. • Anakinra treatment leads to a modest reduction in the signs and symptoms of moderately to severely active rheumatoid arthritis Monoclonal Against cytokine 1. Anti-TNF reagents (Infliximab, Adalimumab) • Mechanism: Binds to TNF-α → prevent binding of TNF-α to its receptor → inhibits its pro-inflammatory effects • used in rheumatoid arthritis. Soluble human TNF- receptor Fused to Fc domain of human IgG Binds to TNF- Prevents interaction of TNF- with its receptors APC Destruction of T lymphocytes TH Inhibit IL-2 synthesis IL-2 Calcineurin inhibitors Cyclosporine - Tacrolimus Block IL-2 receptors mTOR inhibitors Sirolimus and everolimus Block cytokine stimulated cell proliferation - Azathioprine Mycophenolate mofetil Antithymocyte globulin Muromonab (Daclizumab-Basiliximab) IL-2 R Cell cycle progression G1-S Inhibit purine synthesis - T cell proliferation Macrophage Anti IL-1 receptor Anakinra - IL-1, TNF-α - Chronic inflammatory tissue injury Anti TNF-α Infliximab, Adalimumab Etanercept