* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Quantum Mechanics
Wheeler's delayed choice experiment wikipedia , lookup
Orchestrated objective reduction wikipedia , lookup
Quantum key distribution wikipedia , lookup
Identical particles wikipedia , lookup
Quantum teleportation wikipedia , lookup
Renormalization group wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Quantum state wikipedia , lookup
Wave function wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
Renormalization wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Particle in a box wikipedia , lookup
History of quantum field theory wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Tight binding wikipedia , lookup
Canonical quantization wikipedia , lookup
EPR paradox wikipedia , lookup
Elementary particle wikipedia , lookup
Atomic orbital wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
Electron configuration wikipedia , lookup
Electron scattering wikipedia , lookup
Hidden variable theory wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Hydrogen atom wikipedia , lookup
Double-slit experiment wikipedia , lookup
Matter wave wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
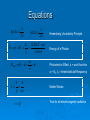
Quantum Mechanics Chapters 27 and 28 The Beginning Thomson-Cathode J. J. Thomson experimented with cathode rays and discovered the charge to mass ratio of the particles that were in there. Thomson called these particles “electrons” Millikan-Oil Ray Experiments Drop Experiment Millikan used a spray atomizer to determine the charge of an electron. This is the smallest charge known and is called the elementary charge Conclusions from Early Experiments Combining Millikan and Thomson’s findings, we are able to determine the mass and charge of an electron. The electron is very, very small-too small to measure accurately Quantized Energy Max Planck measures black body radiation so accurately that he is able to see that it occurs in discrete intervals E = nhf, where h is Planck’s constant, n is an integer, and f is the frequency of the oscillation Discrete is the same as quantum or quantized Photoelectric Effect It was noticed that some metals when incident with certain colors of light would conduct electricity. At the time, it was already widely accepted that light was a wave (Newton thought it was a particle, but Young disproved his theories) According to wave theory, it should not depend on color of light, but rather the intensity Einstein determined that the photoelectric effect is in fact proof that light is a photon (particle) and has energy based on wavelength/frequency, not intensity Wave Nature of Matter De Broglie (pronounce de broy) stated that if light can be both a wave and a particle, maybe all particles can also be waves He was laughed for the most part until the acceptance of quantum mechanics Atomic Spectra Rarefied gases can be excited to emit light Discharge tubes are used that contains very little gas at low pressure A high voltage is applied across the atoms, which cause them to interact to create light (one of the four interactions of photons) For hydrogen, there is an equation for wavelength of light emitted It is able to predict what wavelengths of hydrogen can be found No other element can be predicted as hydrogen can, as the calculations are too complex due to the extra electrons and protons Bohr’s Model of the Atom Niels Bohr had studied with Rutherford (who determined that the nucleus was a concentration of massive particles we now know to be protons and neutrons) and from this made his own model of the atom The Bohr model of the atom explains the equations for the atomic spectrum of hydrogen, but does not work for any other element Even though his theory was wrong, it provided an excellent starting point for quantum mechanics Quantum Mechanics Theory Since we know all of the laws already established hold to be true, it was agreed upon that any laws that are created on an atomic level would correspond to their macroscopic laws The wave function, Y (psi) represents the displacement as a function of time and position Thus, Y2 is the probability of finding a certain electron at the given position and time The Y2 function gives us the shapes of the orbitals Heisenberg Uncertainty Principle Heisenberg stated that it was impossible to measure either the energy of anything at the same instant as time or the momentum at the same instant of position This means that if something is moving, there is a real possibility that you do not know where it actually is This is typically a very small number (10-30) for ordinary objects, but for electrons and other tiny objects it is on the order at which they exist Quantum Numbers Principal-n-energy level, anywhere from 1 to infinity Orbital-l- gives us the shape, can be 0 to n-1 Magnetic-ml-gives us the orientation, can be –l to +l Spin-ms –gives the sign of the angular momentum, can be +1/2 or -1/2 Pauli Exclusion Principle states that no two electrons can have the same set of quantum numbers Equations h p x 2 E t h 2 1240 eV nm E pc hf (nm) hc K max hf h h p mv c f hc Heisenberg Uncertainty Principle Energy of a Photon Photoelectric Effect, = work function = hf0, f0 = threshold/cutoff frequency Matter Waves True for all electromagnetic radiation Problems Chapter 27: pp 782-785 Questions: 5, 6, 7, 25, 26 Problems: 15, 17, 21, 23, 27 Demonstrate: Ex 27-10 on page 766, Ex 27-11 on page 767, P #14 on page 783, Review Graph on page 760, Ex 27-3 and 27-4 on page 761, P #20, 22, 26 on page 783 and GP#84 on page 785 Problems Chapter 28 Questions: 7 Problems: none Demonstrate: none