* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Drosophila melanogaster wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Adaptive immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
DNA vaccination wikipedia , lookup
Molecular mimicry wikipedia , lookup
Duffy antigen system wikipedia , lookup
X-linked severe combined immunodeficiency wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
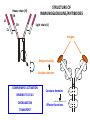
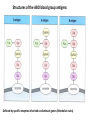
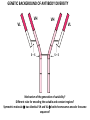
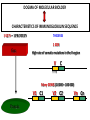
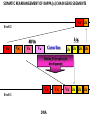
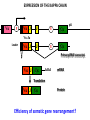
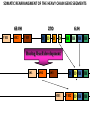
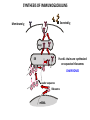
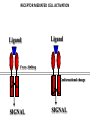
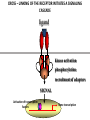
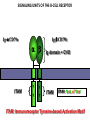
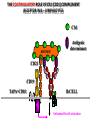
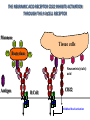
PRODUCTION OF IMMUNOGLOBULINS BEFORE BIRTH AFTER BIRTH breast milk IgA 100% (adult) maternal IgG IgM IgG IgA 0 3 month 6 9 1 2 3 4 5 adult year Ig. Concentration level of antibodies secondary response against Szekunder ’lasyecondary resp antigen A primary response against antigen A response primer IgG IgA IgE IgM IgM primary response against antigen B 5 „A” antigA én Antigen 10 15 20 25 30 „A” és „B” Antigen A and B antigén napok days napok Polyclonal antibody response Ag Polyclonal antibody Immunserum Set of B-cells Ag Activated B-cells Antibodyproducing plasma-cells Antigen-specific antibodie Ig isotype Serum concentration Characteristics, functions Trace amounts Major isotype of secondary (memory) immune response Complexed with antigen activates effector functions (Fc-receptor binding, complement activation The first isotype in B-lymphocyte membrane Function in serum is not known Trace amounts Major isotype in protection against parasites Mediator of allergic reactions (binds to basophils and mast cells) 3-3,5 mg/ml Major isotype of secretions (saliva, tear, milk) Protection of mucosal surfaces 12-14 mg/ml 1-2 mg/ml Major isotype of primary immune responses Complexed with antigen activates complement Agglutinates microbes The monomeric form is expressed in B-lymphocyte membrane as antigen binding receptor STRUCTURE OF IMMUNOGLOBULINS/ANTIBODIES Heavy chain (H) VH VL CH Light chain (L) CL Antigen Antigen binding antigénkötés s VL s s s s s DEGRADATION TRANSPORT s ss ss Constans domains konsta ns dom ének effektor funkc iók Effector functions CH2 ss s s CH3 ss s s s BINDING TO CELLS s s CL s COMPLEMENT ACTIVATION s CH1 s va riábilis d om ének Variable domains s s S s S s s s s VH Antibodies with different isotypes differ in their Binding affinity, effector functions and their Transport. Carbohydrate antigens are usually recognized By IgM type antibodies. Differences in transport makes all the differece: Antibodies spec. to blood group antigens Structures of the ABO blood group antigens Defined by specific enzymes inherited co-dominant genes (Mendelian rules) Donors and recipients for blood transfusion - + + + - - + + - + - + - - - - Rhesus (Rh) blood group antigen (D) POLYPEPTIDE TYPE ANTIGEN extracellular space cytoplasm membrane intracellular space IgG type antibody - incomplete no direct agglutination but human immunglobulin-reactive 2. antibody can cause agglutination indirect agglutination Pathological consequences of placental transport of IgG (hemolytic disease of the newborn) Effects of agglutination in vivo ABO incompatibility intravascular haemolysis (complement mediated haemolysis) Rh incompatibility haemolytic disease of the newborn (erythroblastosis fetalis) (opsonisation of red blood cells, which are then phagocytosed by macrophages and granulocytes) Rh profilaxis ANTIBODY DIVERSITY AMINO ACID SEQUENCE OF IMMUNOGLOBULINS Multiple myeloma (MM) Plasma cell tumors – tumor cells reside in the bone marrow Produce immunoglobulins of monoclonal origin, serum concentration 50-100mg/ml Rodney Porter & Gerald Edelman 1959 – 1960 myeloma protein purification Gel electrophoresis L H Reduction 50 kDa Heavy chain 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 25 kDa Light chain Variable Constant GENETIC BACKGROUND OF ANTIBODY DIVERSITY VH VL S–S VH VL S–S Mechanism of the generation of variability? Different rules for encoding the variable and constant regions? Symmetric molecule two identical VH and VL both chromosomes encode the same sequence? DOGMA OF MOLECULAR BIOLOGY CHARACTERISTICS OF IMMUNOGLOBULIN SEQUENCE 1 GEN = 1 PROTEIN THEORIES 1 GEN Gen High rate of somatic mutations in the V-region V C Many GENES (10 000 – 100 000) V1 C1 Protein V2 C2 Vn Cn MOLECULAR GENETICS OF IMMUNOGLOUBLINS How can the bifunctional nature of antibodies be explained genetically? In 1965, Dreyer & Bennett proposed that for a single isotype of antibody there may be: • A single C region gene encoded in the GERMLINE and separate from the V region genes • Multiple choices of V region genes available • A mechanism to rearrange V and C genes in the genome so that they can fuse to form a complete Immunoglobulin gene. This was genetic heresy as it violated the then accepted notion that DNA was identical in every cell of an individual The Dreyer - Bennett hypothesis V V V V V V V V V V V V C V V A single C region gene is encoded in the germline and separated from the multiple V region genes C A mechanism to rearrange V and C genes in the genome exists so that they can fuse to form a complete Immunoglobulin gene Find a way to show the existence of multiple V genes and rearrangement to the C gene Approach V V V V V V V V V V V V V C Germline DNA C Rearranged DNA V Tools: • A set of cDNA probes to specifically distinguish V regions from C regions • DNA restriction enzymes to fragment DNA • Examples of germline (e.g. placenta) and mature B cell DNA (e.g. a plasmacytoma/myeloma) Experiment of Susumi Tonegawa 1975 Basel DNA-extraction Digestion by restriction enzyme Gel electrophoresis Southern blot Kb Liver cell V-probe 6,0 4,0 1,5 V C-probe C C 6.0 Kb V 4.0 Kb V C 1.5. Kb V C B-cell B-cell CONCLUSION V and C genes get close to each other in B-cells only V V V C B-CELL There are many variable genes but only one constant gene V V V V C GERM LINE Ig gene sequencing complicated the model The structures of germline VL genes were similar for Vk, and Vl, However there was an anomaly between germline and rearranged DNA: VL CL ~ 95aa ~ 100aa L L VL CL ~ 208aa Where do the extra 13 amino acids come from? L VL ~ 95aa JL CL ~ 100aa Some of the extra amino acids are provided by one of a small set of J or JOINING regions Further diversity in the Ig heavy chain L VH DH JH CH The heavy chain was found to have further amino acids (0 – 8) between the JH és CH genes D (DIVERSITY) region Each heavy chain requires 2 recombination events JH to DH , VH to JHDH, L VL JL CL Each light chain requires 1 recombination events VL to JL IMMUNOGLOBULIN CHAINS ARE ENCODED BY MULTIPLE GENE SEGMENTS ORGANIZATION OF IMMUNOGLOBULIN GENE SEGMENTS Chromosome 2 kappa light chain gene segments Chromosome 22 lambda light chain gene segments Chromosome 14 heavy chain gene segments HOW MANY IMMUNOGLOBULIN GENE SEGMENTS Gene segments Light chain kappa Heavy chain lambda Variable (V) 40 30 65 Diversity (D) 0 0 27 Joining (J) 5 4 6 SOMATIC REARRANGEMENT OF KAPPA (κ) CHAIN GENE SEGMENTS Vκ Jκ B-cell 2 Vκ Vκ 5 Jκ 40 Vκ Vκ Vκ Germ line Jκ Jκ Jκ Jκ During B-lymphocyte development Vκ B-cell 1 DNA Vκ Vκ Jk Jκ Jκ Jκ EXPRESSION OF THE KAPPA CHAIN Vκ P Vκ J pA J E Cκ J E Cκ Vκ-Jκ Leader Vκ J Primary RNA transcript Vκ J Cκ AAAA mRNA Translation Vκ J Cκ Protein Efficiency of somatic gene rearrangement? SOMATIC REARRANGMENT OF THE HEAVY CHAIN GENE SEGMENTS 65 VH VH1 VH2 27 D VH3 D D D 6 JH D JH JH JH JH During B-cell development VH1 VH2 VH3 VH1 D D JH JH VH2 D D JH JH VARIABILITY OF B-CELL ANTIGEN RECEPTORS AND ANTIBODIES B cells of one individual 2 3 1 4 V-Domains C-Domains VH D JH VL VH-D-JH JL VL-JL ORDER OF REARRANGEMENTS OF IMMUNOGLOBULIN GENE SEGMENTS D – J recombination V – DJ recombination VDJ – δ transcription Surrogate light chain V – J recombination VJ – k (or VJ - l) transcription δ translation k or l translation B-sejt mIgD mIgM Secreted IgM Estimates of combinatorial diversity Taking account of functional V D and J genes: 65 VH x 27 DH x 6JH = 6,480 combinations 40 Vk x 5 Jk = 200combinations 30 Vl x 4 Jl = 120 combinations = 320 different light chains If H and L chains pair randomly as H2L2 i.e. 6,480 x 320 = 2,073,600 possibilities Due only to COMBINATORIAL diversity In practice, some H + L combinations do not occur as they are unstable Certain V and J genes are also used more frequently than others. There are other mechanisms that add diversity at the junctions between genes - JUNCTIONAL diversity GENERATES A POTENTIAL B-CELL REPERTOIRE THE RESULT OF SOMATIC GENE REARRANGEMENTS 1. Combination of gene segments results in a huge number of various variable regions of the heavy and light chains expressed by different B-cells SOMATIC GENE REARRANGEMENT 2. Successful somatic rearrangement in one chromosome inhibits gene rearrangement in the other chromosome ALLELIC EXCLUSION 3. One B-cell produces only one type of heavy and one type of light chain COMMITMENT TO ONE TYPE OF ANTIGEN BINDING SITE 4. The B-cell pool consist of B-cells with differently rearranged immunoglobulin genes INDEPENDENT OF ANTIGEN OCCURS DURING B-CELL DEVELOPMENT IN THE BONE MARROW Evidence for allelic exclusion ALLOTYPE- a polymorphism in the Heavy chain C region of Ig Allotypes can be identified by staining B cell surface Ig with antibodies B a AND b b B B Y Suppression of H chain rearrangement by pre-B cell receptor prevents expression of two specificities of antibody per cell B Y b Y B Y a a/b b/b Y Y a/a a Allelic exclusion is needed for efficient clonal selection Antibody S. typhi S. typhi All daughter cells must express the same Ig specificity otherwise the efficiency of the response would be compromised Suppression of H chain gene rearrangement helps to prevent the emergence of new daughter specificities during proliferation after clonal selection Allelic exclusion prevents unwanted responses One Ag receptor per cell IF there were two Ag receptors per cell Y Y Y S. aureus Y Y Y Y Anti S. aureus Antibodies B S. aureus Anti brain Abs Y Y Y Y Self antigen expressed by e.g. brain cells Y Y B Anti S. aureus Antibodies Suppression of H chain gene rearrangement ensures only one specificty of Ab expressed per cell. Prevents induction of unwanted responses by pathogens Allelic exclusion is needed to prevent holes in the repertoire One specificity of Ag receptor per cell IF there were two specificities of Ag receptor per cell Anti-brain Ig Anti-brain Ig AND anti-S. Aureus Ig B B Exclusion of anti-brain B cells i.e. self tolerance B Deletion OR B BUT anti S.Aureus B cells will be excluded leaving a “hole in the repertoire” Anergy B S. aureus SYNTHESIS OF IMMUNOGLOBULINS Secreted Ig Membrane Ig Golgi ER H and L chains are synthesized on separated ribosomes CHAPERONES Leader sequence Ribosome mRNA B – CELL ACTIVATION RECEPTOR MEDIATED CELL ACTIVATION Ligand Ligand Cross - linking Conformational change SIGNAL SIGNAL CROSS – LINKING OF THE RECEPTOR INITIATES A SIGNALING CASCADE ligand kinase activation phosphorylation recruitment of adaptors SIGNAL Activation of transcription factors Gene transcription (review) BCR signaling SIGNALING UNITS OF THE B-CELL RECEPTOR Ig-a/CD79a Ig-b/CD79b a b Y ITAM Y Ig domain + CHO Y Y ITAM ITAM: YxxL x7 YxxI ITAM: Immunoreceptor Tyrosine-based Activation Motif KINETICS OF LYMPHOCYTE ACTIVATION ANTIGEN SIGNAL1. Resting limfocita lymphocyteGG Nyugvó 00 Co-receptor Adhesion molecule Cytokines SIGNAL2. Effector cell Memory cell G1 Transport Membrane change RNA and protein synthesis M proliferation G2 S DNA synthesis Lymphoblast Resting lymphocyte G0 PTK activation RNA synthesis Free Ca++ Protein synthesis Protein phosphorylation DNA synthesis 0 10sec 1min 5min 1hr 6 hrs 12 hrs 24 hrs THE CO-STIMULATORY ROLE OF CR2 (CD21) COMPLEMENT RECEPTOR IN B – LYMPHOCYTES C3d ANTIGEN Antigenic determinant CD21 CD19 TAPA=CD81 B-CELL Y Y Enhanced B-cell activation THE NEURAMIC ACID RECEPTOR CD22 INHIBITS ACTIVATION THROUGH THE A B-CELL RECEPTOR Mannose Tissue cells Bacterium Neuraminic (sialic) acid Antigen B Cell CD22 Inhibited B cell activation B cell differentiation is „helped” by T-cells T SEJT (CD4+ helper) ANTIGÉN CITOKINEK B SEJT PLAZMA SEJT IZOTÍPUS VÁLTÁS ÉS AFFINITÁS ÉRÉS CSAK T SEJT SEGÍTSÉGGEL MEGY VÉGBE HOGYAN LÁTJÁK A T SEJTEK AZ ANTIGÉNT? 1) I read that monocytes have a phagocytic role. When is it? Don't they need to be activated and become macrophages to be able to phagocyte? 2) When do monocytes differentiate into macrophages? And when do they differentiate into dentritic cells? 3) I saw that sometimes macrophages are considered as innate immunity, and sometimes as acquired immunity What is the difference within the macrophages? (when are they considered as innate, and when as 4) About the phagoctose process: what are ROI and NO? Aren't lysosomes enough to "digest" the antigen? 5) About the structure of the thymus: why is there macrophages and dentritic cells into it? What is their role? 6) The recognition by toll receptors in unavoidable. But these toll receptors are part of the innate immunity. So are they used to attach the antigen to phagocytose it. And then, the antigen presentation enable the acquired immunity to make a receptor/antibody more precise for this specific antigen? 7) About the cytokines. I read that the IFN gamma are produced by T helper, and they activate the macrophages. Aren't the macrophages activated before? 8) Finally, I didn't understand few slides: Lecture 3-4: slides 31, 43, 44, 45