* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ARVO 2004 poster file
Gene expression programming wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Point mutation wikipedia , lookup
Minimal genome wikipedia , lookup
Primary transcript wikipedia , lookup
Genome (book) wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Designer baby wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Gene expression profiling wikipedia , lookup
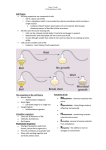
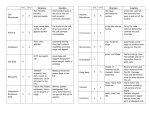
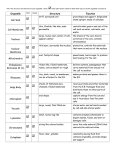
DIFFERENCES IN GENE EXPRESSION PROFILE OF CULTURED ADULT VERSUS IMMORTALIZED HUMAN RPE Lee Geng, Hui Cai and Lucian V. Del Priore Department of Ophthalmology, Columbia University, New York, New York Genes expressed in ARPE cells but not detected in adult RPE cells Table I. Genes expressed in pRPE but not detected in aRPE Purpose: Immortalized human RPE (cell line ARPE-19) are used widely to draw A. inferences about the behavior of adult RPE. We usedGeneDNA microarray analysis to compare the Gene have Title Symbol Functions p21/Cdc42/Rac1-activated kinase 1 p21 (CDKN1A)-activated kinase 2 Down syndrome critical region gene 1-like 1 superoxide dismutase 2, mitochondrial dipeptidylpeptidase 4 neuronal pentraxin II serine (or cysteine) proteinase inhibitor cyclin D2 ribonuclease, RNase A family chondroitin sulfate proteoglycan 2 (versican) DEAD (Asp-Glu-Ala-Asp) box polypeptide 3, Y-linked prostaglandin D2 synthase 21kDa (brain) transmembrane 4 superfamily member 3 alpha-fetoprotein keratin 19 ATPase, Ca++ transporting, plasma membrane 4 prostaglandin I2 (prostacyclin) synthase transferrin receptor (p90, CD71) PAK1 PAK2 DSCR1L1 SOD2 DPP4 NPTX2 SERPINB8 CCND2 RNASE1 CSPG2 DDX3Y PTGDS TM4SF3 AFP KRT19 ATP2B4 PTGIS TFRC protein kinase activity protein kinase ;ATP binding ;transferase activity calcium-mediated signaling response to oxidative stress proteolysis and peptidolysis; immune response heterophilic cell adhesion;regulation of synapse serine protease inhibitor activity regulation of cell cycle;cytokinesis RNA binding ;endonuclease activity heterophilic cell adhesion; cell recognition DEAD;ATP binding prostaglandin biosynthesis; transport protein amino acid glycosylation;pathogenesis transport;immune response structural constituent of cytoskeleton cation transport;calcium ion transport;metabolism prostaglandin biosynthesis;electron transport;lipid metabolism proteolysis and peptidolysis; iron ion transport;endocytosis Methods: Cultured primary RPE from five human donors (age: 48 - 80 years) and ARPE-19 cultured to confluence in five dishes were used for DNA microarray study. Total RNA was B. Genes expressed in Table adult RPE cells but not detected in aRPE-19 cells II. Selected Genes expressed in aRPE but not in pRPE Figure 2. A. a scatter plot of expression levels of about 6,000 genes in pRPE vs. aRPE-19 shows an incomplete overlap in the gene expression profiles of these two cells types. B. Figure shows the distribution of differentially (1.5-fold) expressed genes in pRPE and aRPE-19 cell types Numbers of Gene Expressed in RPE cells 7000 6000 Number of Genes Results: 5000 4000 3000 2000 ARPE 1000 0 pRPE primary RPE A. B. Figure 1. Principle Component Analysis (A) and hierarchic clustering analysis (B) demonstrate that the gene expression profile of the adult RPE (shown in blue color group) and ARPE-19 ( shown in red color group) cluster into two distinct groups with no discernable overlap. ARPE-19 Figure 3. The expression of 5,932 genes (out of 12,600 genes on microarray Human 95UA chip) was detected in ARPE-19 cells, in comparison to expression of only 4,849 genes in adult RPE cells from all 5 human donor eyes aRPE-19 express ubiquitin specific protease 6 (Tre-2 oncogene) BCL2 binding component 3 calcium/calmodulin-dependent protein kinase I death associated transcription factor 1 basic leucine zipper nuclear factor 1 (JEM-1) nicotinamide nucleotide adenylyltransferase 2 transcription factor AP-2 alpha (activating enhancer binding protein 2 alpha) protein kinase C, alpha ubiquitin-conjugating enzyme E2M (UBC12 homolog, yeast) TNF receptor-associated factor 2 interleukin 12A (p35) phosphate cytidylyltransferase 2, ethanolamine zinc finger protein 278, short isoform paraneoplastic antigen programmed cell death 11 leucine-rich repeats and immunoglobulin-like domains 1 ubiquitin specific protease 52 E1A binding protein p400 zinc finger protein 205 small nuclear RNA activating complex, polypeptide 4, 190kDa zinc finger protein 44 (KOX 7) ADP-ribosylation factor related protein 1 Rho guanine nucleotide exchange factor (GEF) 12 phosphatidylserine receptor dead ringer-like 1 (Drosophila) MHC class I polypeptide-related sequence B SH3 domain binding glutamic acid-rich protein nuclear factor of kappa light polypeptide gene enhancer transcription termination factor, RNA polymerase I zinc finger protein 282 peroxisomal acyl-CoA thioesterase protein kinase, cAMP-dependent, regulatory, type II, beta guanidinoacetate N-methyltransferase leucine rich repeat neuronal 4 mitogen-activated protein kinase 8 interacting protein 3 myeloid/lymphoid or mixed-lineage leukemia 4 interleukin 1 receptor accessory protein heat shock 70kDa protein 4 zinc finger protein 23 (KOX 16) elongation of very long chain fatty acids (FEN1/Elo2, SUR4/Elo3, yeast)-like 2 polymerase (DNA directed), gamma 2, accessory subunit teratocarcinoma-derived growth factor 3, pseudogene zinc finger, DHHC domain containing 18 mitogen-activated protein kinase kinase 5 p53-associated parkin-like cytoplasmic protein caspase recruitment domain family, member 10 cell growth regulator with EF hand domain 1 adrenergic, beta-2-, receptor, surface P450 (cytochrome) oxidoreductase USP6 BBC3 CAMK1 DATF1 BLZF1 NMNAT2 TFAP2A PRKCA UBE2M TRAF2 IL12A PCYT2 --HUMPPA PDCD11 LRIG1 USP52 EP400 ZNF205 SNAPC4 ZNF44 ARFRP1 ARHGEF12 PTDSR DRIL1 MICB SH3BGR NFKBIL1 TTF1 ZNF282 PTE1 PRKAR2B GAMT LRRN4 MAPK8IP3 MLL4 IL1RAP HSPA4 ZNF23 ELOVL2 POLG2 TDGF3 ZDHHC18 MAP2K5 PARC CARD10 CGREF1 ADRB2 POR protein modification /// deubiquitination /// oncogenesis --protein amino acid phosphorylation /// signal transduction regulation of transcription, DNA-dependent /// apoptosis ------cell cycle; cell proliferation;induction of apoptosis ubiquitin cycle protein complex assembly /// apoptosis /// signal transduction immune response phospholipid biosynthesis /// biosynthesis ----rRNA processing --UCH;cysteine-type endopeptidase activity;3.3e-07 SNF2_N;DNA binding;1.4e-58 regulation of transcription, DNA-dependent transcription from Pol II promoter regulation of transcription, DNA-dependent signal transduction PDZ;intracellular signaling cascade;1.5e-10 --regulation of transcription, DNA-dependent response to stress /// cellular defense response protein complex assembly --transcription termination regulation of transcription, DNA-dependent lipid metabolism /// acyl-CoA metabolism protein phosphorylation;intracellular signaling cascade creatine biosynthesis /// muscle contraction neurogenesis vesicle-mediated transport /// regulation of JNK cascade regulation of transcription, DNA-dependent; apoptosis ----regulation of transcription, DNA-dependent fatty acid biosynthesis DNA replication /// DNA repair /// protein biosynthesis ----signal transduction --apoptosis;activation of NF-kappaB-inducing kinase --activation of MAPK /// receptor mediated endocytosis electron transport Conclusions: There are some similarities but significant differences in the gene expression profile of cultured adult and immortalized ARPE cells, and it is important to note that some specific genes are only expressed in one of these two groups. These studies suggest caution should be exercised when generalizing results obtained from ARPE-19 to results that would be obtained with adult RPE. Supported by Research to Prevent Blindness, Robert L. Burch III Fund, and the Foundation Fighting Blindness AGING OF BRUCH’S MEMBRANE DECREASES RETINAL PIGMENT EPITHELIUM (RPE) PHAGOCYTOSIS Reiko Koyama, Hui Cai and Lucian V. Del Priore Department of Ophthalmology, Columbia University, NY, New York Methods: Explants of human Bruch’s membrane were prepared as previously described. Donor ages(young eyes: ages 33 - 44; older group: 73 -94). 1-3 Native RPE were removed by bathing the choroid-BM-RPE complex with 0.02 N ammonium hydroxide followed by washing in PBS. The choroid-BM complex was set on polytetrafluoroethylene membrane with the basal lamina of the RPE facing the membrane. 4% agarose was poured onto the choroid -BM- complex from choroidal side and the tissue was kept at 4oC to solidify the agarose. The polytetrafluoroethylene membrane was peeled away and 6 mm circular buttons were trephined from choroid-BM-gel complex. Buttons were placed on 4% agarose at 37oC in non-treated polystyrene wells of a 96 well plate (Figure1). 50,000 immortalized ARPE-19 were seeded onto wells containing Bruch’s membrane explants and bare control wells (plastic only) for 72 hours(5 wells for each age group). 1ul of fluorescent latex beads (3.6x105beads/ul) were added to each well for another 24 hours. ARPE-19 were passaged by trypsinization. Ingested beads were counted using a FACS Flow Cytometer. Data were generated with at least three independent experiments. 30 p<0.02 20 10 cells ingesting beads cells ingesting beads 30 p>0.05 20 10 0 young 400 p<0.0003 300 200 100 older age of Bruch's membrane Fig.4 Percentage of RPE cells which have the ability to ingest beads on younger Bruch’s membrane versus older Bruch’s membrane were similar (25.74±15.94% vs. 22.22±11.59%). Flourescent intensity/cell B. on older Bruch’s membrane cells not ingesting beads plastic Fig.3 Percentage of RPE cells that ingest beads was higher for RPE cultured onto Bruch’s membrane explant than on bare plastic wells (22.22±11.59% vs. 10.04±3.52%). Fluorescent intensity/cell cells not ingesting beads 0 Bruch's Results: A. on younger Bruch’s membrane 40 % of RPE ingesting beads % of RPE ingesting beads Purpose: The earliest changes in age-related macular degeneration occur within Bruch’s membrane. Bruch’s membrane aging affects the attachment and survival of the overlying RPE.1-3 Herein we determine the effects of Bruch’s membrane aging on RPE phagocytosis ability. 400 p<0.008 300 200 100 0 0 Bruch's plastic young old age of Bruch's membrane fluorescent intensity Fig 1. Photo shows 6-mm circular buttons which were trephined from BM and placed into 96 well plate fluorescent intensity Fig.2. Flow cytometry histogram demonstrating the distribution of fluorescent intensity. RPE cell population which ingest fluorescent beads (green zone) in young BM group (A) is larger than older BM group (B). References: 1. Tezel TH, Del Priore LV, Kaplan HJ. Fate of Human Retinal Pigment Epithelial Cells Seeded onto Layers of Human Bruch’s Membrane. Invest Ophthalmol Vis Sci 1999;40:467-476. 2. Tezel TH, Del Priore LV. Repopulation of Different Layers of Host Human Bruch’s Membrane by Retinal Pigment Epithelial Cell Grafts. Invest Ophthalmol Vis Sci 1999;40:767-774. 3. Del Priore LV, Tezel TH. Reattachment Rate of Human Retinal Pigment Epithelium to Layers of Human Bruch’s Membrane. Arch Ophthalmol 116;335-341, 1998. Supported by Research to Prevent Blindness, Robert L. Burch III Fund, and the Foundation Fighting Blindness Supported by Research to Prevent Blindness, Robert L. Burch III Fund, and the Foundation Fighting Blindness. Fig. 5. Comparison of the phagocytosis ability of RPE cells cultured on young Bruch’s membrane explant versus older BM. Average fluorescent intensity per cell (measure of capacity of phagocytosis per cell) on Bruch’s membrane was higher than on plastic (291.61±80.91 vs. 167.50±35.01). Fig. 6 Observation of the population of cells that had ingested beads. The average fluorescent intensity per cell, which is a measure of capacity of phagocytosis per cell in the younger Bruch’s membrane group was higher than on older Bruch’s membrane (312.60±83.80 vs. 267±71.08) Conclusions: This study suggests that Bruch’s membrane promote RPE phagocytosis compared to bare plastic tissue culture wells, and aging of Bruch’s membrane reduces the ability of RPE to ingest beads. To our knowledge, this is the first demonstration that aging of Bruch’s membrane can modulate RPE phagocytosis ability. Further study is required to determine the implications of this age-dependent decrease in RPE phagocytosis in the pathogenesis of AMD. BRUCH'S MEMBRANE AGING ALTERS THE RPE EXPRESSION PROFILE OF PROLIFERATION, MIGRATION AND APOPTOSIS BUT NOT ANGIOGENESIS GENES Hui Cai, Lucian V. Del Priore Department of Ophthalmology, Columbia University, New York, New York PROLIFERATION GENES A. B. OVERALL Purpose: Principal component analysis (PCA) is a technique used to determine global changes in gene expression in response to changing cellular conditions. We have used PCA to YOUNG BM 22.0% OLD BM PC#2 PC#2 18.3% EXPRESSION Methods: Immortalized human ARPE-19 cells were seeded onto human BM (five young samples: donor age = 31- 47 yr and five older samples: donor age = 71 – 81 years) harvested fr PC#1 27.4% C. PC#1 D. PC#2 Results: ANGIOGENESIS GENES PC#2 21.0% 18.4% MIGRATION GENES 47.2% 14000 PC#1 PC#1 35/2% 33.1% 12000 on 38 yo BM 10000 8000 6000 4000 2000 0 0 2000 4000 6000 8000 10000 12000 14000 on 31 yo BM Figure 1. The expression of approximately 6,000 genes (out of 12,600 genes on microarray Human 95UA chip) was detected. Scatter plot of gene expression within RPE cultured onto 31 year-old vs 38 year-old Bruch’s membrane. More than 96% of genes are expressed consistently among all samples tested within the young age group (data not shown). The correlation co-efficient is 0.989 suggesting limited variation between these individuals. Figure 2. Principal component analysis of gene expression. (A) The pattern of gene expression of human RPE seeded onto Bruch’s membrane explants from younger donors (blue) shows a tighter clustering than the gene expression profile of RPE seeded onto older donors (red) Bruch’s membrane (B) Cell proliferation and migration genes of RPE seeded onto young Bruch’s membrane (blue) show a tight clustering with spread in the expression profile of proliferation-related genes of human RPE seeded onto older Bruch’s membrane explants (red). (C) There was a similar pattern for apoptosis-related and genes . (D) There is no age-dependent alteration in the spread in the expression profile of angiogenesis genes. Probe Set ID 160025_at 1933_g_at 31886_at 33377_at 348_at 35016_at 36197_at 36310_at 36916_at 36924_r_at 37393_at 38489_at 38957_at 39171_at 39375_g_at 39771_at 40257_at 40641_at 41119_f_at 41479_s_at Gene Name transforming growth factor, alpha ATP-binding cassette, sub-family C (CFTR/MRP) 5'-nucleotidase, ecto (CD73) vitronectin (serum spreading factor) kinesin family member C1 CD74 antigen (invariant polypeptide of MHC) catilage GP-39 protein keratin, hair, acidic, 1 sialyltransferase 4C secretogranin II (chromogranin C) hairy and enhancer of split 1 heparin-binding growth factor binding protein doublecortin and CaM kinase-like 1 catenin, beta interacting protein 1 G-2 and S-phase expressed 1 Rho-related BTB domain containing 1 Homo sapiens clone 24649 mRNA sequence BTAF1 RNA polymerase II Homo sapiens, clone IMAGE:4310637 RAD51 homolog C Microarray old/young P value TGFA 1.7 0.0031 ABCC5 0.5 0.0058 NT5E 1.7 0.0057 VTN 0.4 0.0049 KIFC1 1.6 0.0074 CD74 1.5 0.0072 Y08374 0.4 0.0016 KRTHA1 1.5 0.0041 SIAT4C 1.6 0.0066 SCG2 0.4 0.0059 HES1 0.5 0.0106 HBP17 2.4 0.0036 DCAMKL1 0.4 0.0010 CTNNBIP1 0.5 0.0041 GTSE1 1.5 0.0091 RHOBTB1 0.5 0.0002 Al400011 1.9 0.0093 BTAF1 1.7 0.0105 W27452 1.7 0.0088 0.0091 RAD51C 1.5 Symbol qRT-PCR old/young up n/c down n/c n/c n/c down n/c down Table I. 20 genes and EST’s with the lowest p-values. Microarray data suggests that aging of Bruch’s membrane increases the expression level of numerous genes. We performed RT-PCR on several genes of interest, including up regulated genes transforming growth factor alpha, CD74 antigen, and heparin-binding growth factor binding protein, and down regulated genes that include the ATP-binding cassette, vitronectin, cartilage GP-39 protein, doublecortin and CaM kinase-like 1, and catenin. RT-PCR confirms the up regulation of TGF alpha and the down regulation of vitronectin, doublecortin and CaM kinase-like 1, and Rhorelated BTB domain containing 1. Conclusions: Age-related changes within BM alone induce significant spreading of the gene expression profile of proliferation, apoptosis and cell migration genes, with no change in angiogenesis genes. These observations suggest some of cellular changes that develop within the RPE as a function of age, such as occur in age-related macular Supported by Research to Prevent Blindness, Robert L. Burch III Fund, and the Foundation Fighting Blindness degeneration, may be the result of substrate-induced alterations in the behavior of the overlying RPE. RPE EXPRESSION OF VITRONECTIN AND ITS RECEPTOR ARE DOWNREGULATED WITH AGING OF HUMAN BRUCH’S MEMBRANE L. V. Del Priore, H. Cai, and T. H. Tezel Department of Ophthalmology, Columbia University, New York, New York Purpose: Previous studies shows that vitronectin is a major constituent of ocular drusen and vitronectin mRNA is synthesized in RPE cells. The purpose of this study is to determine if Bruch’s VN and its receptor expression level in RPE cultured on different aged BM Figure 2. DNA microarray data show vitronectin (VN) and its receptor alphaV 500 transcript expression patterns. ARPE –19 cells 400 were cultured on different aged Bruch’s VN receptor VNseeded onto Methods: DNA microarray and semi-quantitative RTPCR method were used for this study. Immortalized human ARPE-19 cells were human Bruch’s (five samples from don membrane explants for 72membrane hours. Data show 300 VN and its receptor subunit alphaV expression 200 level decreases in RPE cells overlaying on aged 100 Bruch’s membrane. Relative VN expression level 600 0 young older young older BM donor ages GADPH A. Results: C. A. VN and its receptor expression level in RPE cultured on different ages of BM Relative VN expression level 6 5 VN VN receptor 4 3 2 1 0 31 35 38 47 BM donor age B. 71 76 81 Figure 1. A. Plot of individual DNA microarray data on vitronectin (in red color) and its receptor subunit alphaV (in blue color) transcript expression levels. Data show a general decreased expression level trends for both VN and its receptor mRNA in RPE cells seeded onto aged Bruch’s membrane. B. Heat map shows VN and its receptor expression levels (high level in red color and low level in green) in RPE cells cultured on BM explant from different donor ages. VN RECEPTOR B. VN Figure 3. Real time semi-quantitative RT-PCR. The Bruch’s membrane samples were from different batches of donors than those shown above. (A) Quantitative RT-PCR was performed , after establishing standard curves with GAPDH house-keeping gene using serial dilutions of total RNA. (B) Vitronectin expression level is decreased in RPE cells seeded onto aged Bruch’s membrane (dashed lines in duplicates). (C) vitronectin receptor alphaV subunit mRNA in RPE cells is also decreased upon culturing on aged BM (dashed lines). Conclusions: Aging of Bruch’s membrane downregulates the expression profile of vitronectin mRNA and its receptor in human RPE. The vitronectin receptor may play an important role in phagocytosis of photoreceptor outer segments and vitronectin partially mediates RPE attachment to human Bruch’s membrane. These observations suggest some of the changes seen in age-related macular degeneration may be the result of substrate-induced alterations in the behavior of the overlying RPE. Supported by Research to Prevent Blindness, Robert L. Burch III Fund, and the Foundation Fighting Blindness. ANATOMICAL SIMILARITIES BETWEEN STAGE 3 AND STAGE 4 MACULAR HOLES: IMPLICATIONS FOR TREATMENT Jon Wender, Tomohiro Iida, M.D., Lucian V. Del Priore, M.D., Ph.D. Department of Ophthalmology, Columbia University, New York, NY Design: Cross sectional. Methods: Retrospective study in a clinical practice. The study included 42 eyes of 42 patients, each with a stage 3 or 4 macular hole (Gass classification). Macular holes were staged on the basis of clinical exam, Optical Coherence Tomography, and intraoperative findings. We measured the radius of the macular hole and the radius of the surrounding cuff of subretinal fluid from color or red free fundus photographs, and determined the relationship between these 2 variables. Results: The mean age of the patients was 68.0 7 years old (range 51-80). 25 patients had stage 3 macular holes and 17 patients with stage 4 macular holes. The radius of the neurosensory detachment radius was related to the square of the macular hole radius for stage 3 and stage 4 holes, with no significant difference between the stage 3 and stage 4 linear trend lines (p=0.999). There was no correlation between patient age and the area of the macular hole (r = 0.0645) or neurosensory detachment plus hole (r=0.156) over the range of age in this study (51-80 years). However, the area of the doughnut-shaped cuff of subretinal fluid increased with increasing patient age (p = 0.0493), thus suggesting an age-dependent decline in the pumping ability of the RPE. Conclusions: Our data is consistent with a hydrodynamic model in which macular hole anatomy is determined by a balance between fluid flow through the hole and fluid outflow across the RPE. Since Stage 3 and 4 macular holes exhibit a similar relationship between the size of the macular hole and the size of the cuff of subretinal fluid around the hole, simple relief of vitreomacular traction would not lead to resolution of the subretinal fluid cuff unless it is accompanied by a reduction in hole diameter due to approximation of wound edges. Mathematical Model of Macular Holes For a Newtonian fluid, the rate of fluid flow into the hole is limited by the size of the macular hole itself. Mathematically, fluid flow through the macular hole (Fin) is inversely proportional to the resistance (R) to fluid flow through the hole. Thus, we write: (Eq. 1) Fin = / 1/2r14 = 2r14, where is an arbitrary constant. The flow out (Fout) of the subretinal space is due to active pumping of fluid by the underlying RPE. If we assume that the pumping ability of the RPE is homogeneous (i.e., does not vary across the area of the neurosensory detachment), then outward flow will be directly proportional to the area of the RPE under the macular hole and the surrounding neurosensory detachment. Thus, we write: (Eq. 3) Fout = Kr2 2 where r2 is the radius of the surrounding neurosensory detachment (Fig. 1). A priori, it is not known if K is a constant or varies with patient age. We note this explicitly by writing: (Eq. 4) a1 r2 Fout = K(age) r22 In this model, the subretinal fluid cuff will increase in size until enough RPE is exposed to allow the flow out to balance the fluid inflow through the hole. In equilibrium, (Eq. 5) Fin = Fout (Eq. 6) 2 r14 = Kr22 (Eq. 7) r14 = (K/) r22 (Eq. 8) r12 = (K/) r2 2000000 800 1800000 700 1600000 600 Stage III Stage IV 500 Stage III Stage IV 400 1400000 1200000 1000000 Stage III 800000 Stage IV s Stage III 600000 Stage IV 400000 300 200000 200 a2 0 20000 40000 60000 80000 100000 120000 0 30000 140000 80000 macular hole radius 2 (um 2) F in 180000 230000 280000 330000 380000 430000 Figure 4. Subretinal fluid cuff area (a2-a1) vs. macular hole area (a1) for stage 3 and stage 4 macular holes. There appears to be no significant difference between the 2nd order polynomial trend lines for stage 3 (y = 10-05x2 - 0.7656x + 258038, R2 = 0.6752) vs. stage 4 (y = 10-05x2 - 1.8772x + 426462, R2 = 0.8225) macular holes. 2 Table 1. Patient age (years) vs. a1, a2, a2-a1, and a2 /a1 3000000 F out 130000 m acular hole are a (um 2) Figure 2. Relationship between neurosensory detachment radius (r2) and macular hole radius squared (r12). Data fit to a linear regression model, with no significant difference between the stage 3 (y = 0.0042x + 220.04, R2 = 0.8098) and stage 4 (y = 0.0042x + 243.61, R2 = 0.8046) linear trend lines (p=0.999). Fin 1/R The resistance is proportional to the square of the area of the macular hole, where the area of the macular hole is given by r12. Thus, (Eq. 2) r1 Mean age: 68.0 7 years (range 51-80) • 25 patients with stage 3 macular holes • 17 patients with stage 4 macular holes • The neurosensory detachment radius was related to the square of the macular hole radius for stage 3 and stage 4 holes, with no significant difference between the stage 3 and stage 4 linear trend lines (p=0.999). • Similarly, the neurosensory detachment area was related to the square of the macular hole area for stage 3 and stage 4 holes, with no significant difference between the stage 3 and stage 4 linear trend lines. 2500000 FIGURE 1. (Top) Schematic diagram of fluid dynamics of macular hole with surrounding neurosensory detachment. r1 = radius of macular hole, r2 = radius of subretinal fluid cuff. (Bottom) Diagram illustrating fluid dynamics for macular holes. Fin and Fout represent the fluid flow into and out of the subretinal space, respectively. For a Newtonian fluid, Fin is inversely proportional to the resistance. Fout is proportional to the area of the underlying RPE. Thus, the hydrodynamic model predicts that r12 would be proportional to r2; i.e., as the radius of the macular hole doubles, the radius of the neurosensory detachment would quadruple. Methods Retrospective study in a clinical practice. The study included 42 eyes of 42 patients, each with a stage 3 or 4 macular hole (Gass classification). Macular holes were staged on the basis of clinical exam, Optical Coherence Tomography, and intraoperative findings. We measured the radius of the macular hole and the radius of the surrounding cuff of subretinal fluid from color or red free fundus photographs, and determined the relationship between these 2 variables. neurosensory detachment area (um 2) Our model relies on the fact that there is flow of fluid from the vitreous cavity, across the intact retina and RPE, into the choroid in the normal human eye. The development of a full thickness defect in the neurosensory retina will allow fluid from the vitreous cavity to flow through the hole and detach the retina from the RPE (Fig. 1). Fluid flow through the hole will create an enlarging neurosensory detachment. The neurosensory detachment around the hole will increase in size and ultimately be limited by the pumping ability of the underlying RPE. In equilibrium (i.e., when there is no further enlargement of the hole), the fluid flow into the hole (Fin) and fluid flow out of the hole through the RPE (Fout) will be equal (Fig. 1). Results subretinal fluid cuff area (um 2) Purpose: To determine whether the observed anatomy of macular holes can be explained by a hydrodynamic model in which fluid flow through the hole is balanced by fluid pumping across the RPE. We use this model to draw conclusions about the possible role of vitreomacular traction in determining the morphology of macular holes and their resolution after vitreous surgery. neurosensory detachment radius (um) ABSTRACT 2000000 Stage III <66 ≥66 p-value 180,000 + 117,000 695,000 + 475,000 514,000 + 383,000 2,970,000 + 2,600,000 227,000 + 144,000 1,090,000 + 839,000 858,000 + 715,000 5,760,000 + 5,420,000 0.267 0.0617 0.0493 0.0296 Stage IV 1500000 Stage III Stage IV 1000000 500000 0 0 50000000000 1E+11 1.5E+11 2 a1 (um ) 2 a2 (um ) 2 a2-a1 (um ) 2 2 a2 /a1 (um ) 2E+11 macular hole area2 (um 2) Figure 3. Relationship between neurosensory detachment area and macular hole area squared. Note that the data now fits a linear regression model with no significant difference noted between stage 3 (y = 10-05 + 275658, R2 = 0.7677) and stage 4 (y = 10-05x + 337788, R2 = 0.8691) linear trend lines (p=0.904). Conclusions Our data is consistent with a hydrodynamic model of macular hole anatomy in which fluid flow through the hole is balanced by the outflow of fluid across the RPE. Since Stage 3 and 4 macular holes exhibit a similar relationship between the size of the macular hole and the size of the cuff of subretinal fluid around the hole, simple relief of vitreomacular traction would not lead to resolution of the subretinal fluid cuff unless it is accompanied by a reduction in hole diameter due to approximation of wound edges.