* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Genome (book) wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Tay–Sachs disease wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Quantitative trait locus wikipedia , lookup
Oncogenomics wikipedia , lookup
X-inactivation wikipedia , lookup
Designer baby wikipedia , lookup
Genetic drift wikipedia , lookup
Skewed X-inactivation wikipedia , lookup
Saethre–Chotzen syndrome wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
Hardy–Weinberg principle wikipedia , lookup
Population genetics wikipedia , lookup
Frameshift mutation wikipedia , lookup
Dominance (genetics) wikipedia , lookup
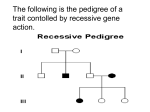
Single Gene Mutations and Inheritance II April 4, 2008 Lisa Schimmenti, M.D. Objectives • • • • Understand autosomal recessive inheritance. Know the concept of Lyonization. Learn and understand the various forms of X linked inheritance. Know how to calculate the risk of affected offspring given a family history and some facts about carrier frequency using the HardyWeinberg Calculation. Autosomal Recessive pattern of inheritance • Males and females equally affected • Condition seen in sibs but not • • • usually in other relatives Risk of recurrence in sibs 25% 2/3 of unaffected sibs are carriers Consanguinity increases risk Autosomal Recessive Probabilities Mother A A AA a Aa a Aa aa 25% affected 2/3 of unaffected are carriers Loss of function alleles • Likely when point mutations give • • the same phenotype as deletion of an allele Inherited as recessive traits when 50% of expression is sufficient for normal phenotype Loss of function and gain of function changes in the same gene will cause different diseases Effect of consanguinity DD DD Dd Dd DD Dd Dd DD Dd DD Dd DD dd Consanguinity increases the risk of sharing a common ancestral mutation Sweeping generalizations about genes inherited as AR traits • • • Transcription from one allele is sufficient Severity dependent on nature and location of mutation What we describe in inheritance pattern as homozygosity is USUALLY compound heterozygosity at molecular level. Allelic heterogeneity is COMMON A common type of AR trait Mutation in enzyme gene • Example: –degradation of mucopolysaccharides requires a series of lysosomal enzymes. –abnormalities in any of these enzymes can cause a similar phenotype: • coarse facies, enlarged organs, skeletal One MPS disease: a-L-iduronidase deficiency • Severe mutations = • Hurler Milder mutations = Scheie http://medgen.genetics.utah.edu / http://www.mpssociety.ca/gallery/ReaganKnight.html Phenotype is dependent on nature of mutation in alleles A family whose child is affected with maple syrup urine disease (MSUD) has sought analysis of their child’s MSUD mutations. MSUD is an enzyme deficiency (inborn error of metabolism) that is inherited in an autosomal recessive pattern. Two mutations are identified in the child’s DNA. The first deletes two base pairs in the coding sequence in exon 1 o f the gene, the second is a T-to-A transversion that alteres a tyrosine to an asparagine at residue 394. W hich of the following statements are TRUE. a. b. c. d. e. The first mutation is likely to be mild because it is “in frame.” Given the nature of the mutations the family is unlikely to have another child that is similarly affected. Neither mutation is likely disease-causing as one yields a frame shift and the other a simple aminoacid substitution. The child is a compound heterozygote for this gene locus. The second mutation is likely to be paternally derived because it is a transversion. Genes in populations How recessive conditions occur in population groups. Deafness • • • • 1 in 1000 infants is born deaf. Infants are screened for deafness/hearing loss at birth by automated hearing screening methods. There are over 400 genes that cause hearing loss Autosomal recessive mutations in GJB2, encoding Connexin 26, will be causative in nearly half of all deaf individuals. Photo credit: Josie Helmbrecht Hearing loss is the most common condition found at birth Gene Mutations in Connexin26 are a common cause of hearing loss Start codon Exon 1 158 bp Exon2 681 bp Protein Six connexons form a hemichannel in one cell membrane. When two cell membranes meet, a gap junction is formed. Connexin 26 • Common deafness alleles – One method for determining carrier frequency • Screen hearing individuals for the presence of the gene • • • mutation. Remember, we have two of each gene Carriers are heterozygotes – one wildtype and one mutant allele 35delG – This is the most common allele in European populations. – How do you determine the carrier frequency without testing hundreds of individuals? Hardy-Weinberg Law • • A mathematical model for calculating allele and gene frequencies in a population Assumes – Large population with random mating – Allele frequencies remain constant • no new mutations • no reproductive selection bias • no significant immigration for populations with different allele frequencies Allele frequencies • p = the frequency of allele 1 • q = the frequency of allele 2 The sum of all alleles for a population is 1 p + q =1 Hardy Weinberg Calculation • Binomial expansion (p+q)2 = p2 + 2pq +q2 p2 = the number of homozygous wildtype individuals in the population 2pq = the number heterozygous carriers in the population q2 = the number of homozygous affected individuals in the population Using the Hardy Weinberg calculation to determine the carrier frequency in a population q2 = affected individuals in the population for Connexin 26 related deafness: q2 =1 in 2000 (an approximated number for this exercise) p2 @ 1 Solve for 2pq Using the Hardy Weinberg calculation to determine the carrier frequency in a population q2 =1 in 2000 = 0.0005 q @ 0.02 2pq = 0.04 The carrier frequency is 0.04 or 1 in 25. • Hardy Weinberg calculations are used in clinic every day Clinical situation: – A 23 year old hearing woman named Marie comes to your genetics clinic. Her family is of European descent Marie has a deaf sister, Sally, who has deafness caused by mutations in the gene encoding Connexin 26. Her sister's genotype is 35delG/35delG. – Marie would like to know her chance of having deaf children caused by mutations in Connexin 26 if the father of the child is of European descent. How do you answer Marie's question? • What is Marie's chance of being a carrier of 35delG? • What is the chance that the father of Marie's child will be a carrier of 35delG? • What is the chance that Marie will have a child with hearing loss? Draw a pedigree Marie Sally What is Marie's chance of being a carrier of 35delG? • Marie's parents are obligate carriers. • Marie is unaffected. • The chance of being a carrier if unaffected is 2/3. Use a Punnett square if in doubt. Wt/35delG Marie Wt/35delG Sally 35delG/35delG What is the chance that the father of Marie's child will be a carrier of 35delG? q2 =1 in 2000 = 0.0005 q @ 0.02 2pq = 0.04 The carrier frequency is 0.04 or 1 in 25. The father's chance of being a carrier is 1/25. What is the chance that Marie will have a child with hearing loss? • The chance that an affected child will be born to a carrier couple is 1 in 4 with each pregnancy. • Calculation: Father of baby's chance of being a carrier • 2/3 x 1/25 x 1/4 = 1/150 Marie's chance of being a carrier Inheritance patterns and genes • Single allele change gives disease – Genes on X (X - linked) – Genes on autosome (AD) • Mutation in both alleles required for disease – Genes on autosome (AR) – (rare) female homozygote for XL X-linked patterns of inheritance • X-linked recessive – Primarily males affected – Females typically unaffected, but there are exceptions • X-linked dominant – Male surviving – Either sex affected, males more severely than females – Male lethal – Only females seen with disease, mosaic patterns of expression X-linkage: already exceptions to the “rules” • The lines of dominant and recessive are blurred • X-linked conditions are sometimes called “semi-dominant” in women • Need dosage compensation Lyonization • Only one X is active in each cell. All others are inactivated. – X inactivation is random – X inactivation is fixed – X inactivation occurs early in development (late blastocyst) XX Lyonization XX XX XX XX XX XX XX XX XX XX X = active X I = inactive X X Inactivation XI IX IX IX IX XI XI IX IX XI Consequences of “Lyonization” • Females are mosaic for their X-linked gene manifestations – should be roughly equal • All are functionally hemizygous – dosage compensation: have the same number of copies as males • Inactive X may be evident in cells – The Barr body • Shifting from random distribution may result in manifestation of disease in females – skewed lyonization X-linked recessive “rules” • Affects mainly males – Affected males are usually born to unaffected parents – Females can be affected if born to an affected father and carrier mother – Females can be affected if they have very skewed lyonization • No male to male transmission • Males born to carrier mother have 50% risk of inheriting altered gene X-linked recessive What does the pattern look like? Carrier female Affected male Normal male Normal female X-linked recessive recurrence risks Mother X1 X1 Y X1X1 X1Y X2 X1X2 Daughters 50% normal 50% carriers X2Y Sons 50% normal 50% affected Duchenne/Becker Muscular Dystrophy • Mutations in dystrophin gene • Duchenne – Typically del/dup but frameshift – Severe – No protein product • Becker – Also del/dup in frame so milder – Shortened protein Clinical manifestations of Duchenne MD http://www.mdausa.org/publications/fa-md-9.html Onset before age 5 Calf pseudohypertrophy 1/3500 males Elevated creatine kinase Severe muscle wasting Affects resp and cardiac mm Females 8-10% some weakness CK > 95%tile in 2/3 of carriers http://www.neuro.wustl.edu/neuromuscular/musdist/lg.html Dystrophin gene and molecule Huge gene (2.3 Mb) 14kb transcript 3685 AAs High mutation rate (10-4) http://www.ncbi.nlm.nih.gov/cgi-bin/SCIENCE96/gene?DMD Using dystrophin antibody normal Becker Duchenne B B n D D http://www.neuro.wustl.edu/neuromuscular/musdist/lg.html X-linked dominant “rules” • Males and females both affected , but typically males are worse than females. • If male lethal, only affected females observed. • Affected males will only have affected daughters and unaffected sons. • No male -> male transmission seen X-linked dominant What does the pattern look like? Affected female Affected male Normal male Normal female Example with male survival X-linked dominant, male lethal What does the pattern look like? Affected female Normal male Normal female X-linked dominant recurrence risks Mother X1 X1 Y X1X1 X1Y X2 X1X2 Daughters 50% normal 50% affected X2Y Sons 50% normal 50% affected Incontinentia pigmenti X-linked dominant (male lethal condition) • • • • • • • Linear blisters in newborn girls “Crops” followed by scarring Small teeth Eye abnormalities Patchy hair loss Only girls are affected Males are typically not affected Infant with IP Linear blisters on leg Linear erosions on soles http://dermatology.cdlib.org/DOJvol4num1/path/incont.html Potentially confusing in X-linked pedigree analysis • Male lethal X-linked conditions • New mutations may be hard to recognize as X-linked • Sex-limited conditions may look Xlinked • With carrier mother and affected father can see “male to male” inheritance Fred Jones is red-green color blind as i s his son, Frank, but Frank’s mom , Freida, is not. However, as an expert in genetics, you know that red-green color blindness is inherited in an X-linked pattern. What is the most likely explanation for the apparent inheritance of this trait in this family? a. Color blindness is a sex-limited trait occurring only in males b. Frank is probably not Fred’s biologic offspring c. Frank has a new mutation for red-green color blindness d. Freida is a carrier (heterozygote) for red-green color blindness e. Males homozygous for X-linked traits usually don’t survive. What is the chance that the next BOY born to this couple will also be red-green color blind? a. 100% because Frank is affected. b. 100% because Freida is a carrier. c. 50% because Fred is affected. d. 50% because Frieda is a carrier. e. 50% because Frank is affected.