* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Enzyme Properties
Fatty acid synthesis wikipedia , lookup
Carbon sink wikipedia , lookup
Microbial metabolism wikipedia , lookup
Proteolysis wikipedia , lookup
Metalloprotein wikipedia , lookup
Photosynthesis wikipedia , lookup
Biosequestration wikipedia , lookup
Blood sugar level wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
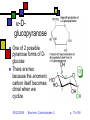
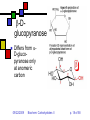
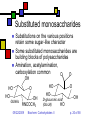
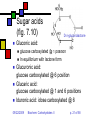
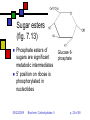
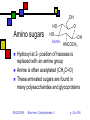
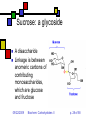
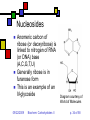
Carbohydrates II Andy Howard Introductory Biochemistry, Fall 2009 22 September 2009 Biochem: Carbohydrates II 09/22/2009 Sugars and polysaccharides Sugars are vital as energy sources, and they also serve as building blocks for lipid-carbohydrate and proteincarbohydrate complexes 09/22/2009 Biochem: Carbohydrates II p. 2 of 58 Plans for Today Sugar Concepts Monosaccharides Cyclization Reducing and nonreducing sugars Sugar Derivatives Oligosaccharides Glycosides 09/22/2009 Polysaccharides Starch & glycogen Cellulose and chitin Glycoconjugates Biochem: Carbohydrates II Proteoglycans Peptidoglycans Glycoproteins p. 3 of 58 Sugar nomenclature All sugars with m ≤ 7 have specific names apart from their enantiomeric (L or D) designation, e.g. D-glucose, L-ribose. The only 7-carbon sugar that routinely gets involved in metabolism is sedoheptulose, so we won’t try to articulate the names of the others 09/22/2009 Biochem: Carbohydrates II p. 4 of 58 Fischer projections Convention for drawing openchain monosaccharides If the hydroxyl comes off counterclockwise relative to the previous carbon, we draw it to the left; Clockwise to the right. 09/22/2009 Biochem: Carbohydrates II Emil Fischer p. 5 of 58 Cyclic sugars Sugars with at least four carbons can readily interconvert between the openchain forms we have drawn and fivemembered(furanose) or six-membered (pyranose) ring forms in which the carbonyl oxygen becomes part of the ring There are no C=O bonds in the ring forms 09/22/2009 Biochem: Carbohydrates II p. 6 of 58 Furanoses Formally derived from structure of furan Hydroxyls hang off of the ring; stereochemistry preserved there Extra carbons come off at 2 and 5 positions 09/22/2009 Biochem: Carbohydrates II 1 5 2 4 3 furan p. 7 of 58 1 Pyranoses 6 Formally derived from structure of pyran Hydroxyls hang off of the ring; stereochemistry preserved there Extra carbons come off at 2 and 6 positions 09/22/2009 Biochem: Carbohydrates II 2 3 5 4 pyran p. 8 of 58 How do we cyclize a sugar? Formation of an internal hemiacetal or hemiketal (see a few slides from here) by conversion of the carbonyl oxygen to a ring oxygen Not a net oxidation or reduction; in fact it’s a true isomerization. The molecular formula for the cyclized form is the same as the open chain form 09/22/2009 Biochem: Carbohydrates II p. 9 of 58 Family tree of aldoses Simplest: D-, L- glyceraldehyde (C3) Add —CHOH: D,L-threose, erythrose (C4) Add —CHOH: D,L- lyxose, xylose, arabinose, ribose (C5) Add —CHOH: D,L-talose, galactose, idose, gulose, mannose, glucose, altrose, allose (C6) 09/22/2009 Biochem: Carbohydrates II p. 10 of 58 Family tree of ketoses Simplest: dihydroxyacetone (C3) Add —CHOH: D,L-erythrulose (C4) Add —CHOH: D,L- ribulose, xylulose (C5) Add —CHOH: D,L-sorbose, tagatose, fructose, psicose (C6) 09/22/2009 Biochem: Carbohydrates II p. 11 of 58 Haworth projections …provide a way of keeping track the chiral centers in a cyclic sugar, as the Fischer projections enable for straight-chain sugars 09/22/2009 Biochem: Carbohydrates II Sir Walter Haworth p. 12 of 58 O The anomeric carbon C In any cyclic sugar (monosaccharide, or single unit of an oligosaccharide, or polysaccharide) there is one carbon that has covalent bonds to two different oxygen atoms We describe this carbon as the anomeric carbon 09/22/2009 Biochem: Carbohydrates II O p. 13 of 58 iClicker quiz, question 1 Which of these is a furanose sugar? 09/22/2009 Biochem: Carbohydrates II p. 14 of 58 iClicker quiz, question 2 Which carbon is the anomeric carbon in this sugar? (a) 1 (b) 2 (c) 5 (d) 6 (e) none of these. 09/22/2009 Biochem: Carbohydrates II p. 15 of 58 iClicker, question 3 How many 7-carbon D-ketoses are there? (a) none. (b) 4 (c) 8 (d) 16 (e) 32 09/22/2009 Biochem: Carbohydrates II p. 16 of 58 a-Dglucopyranose One of 2 possible pyranose forms of Dglucose There are two because the anomeric carbon itself becomes chiral when we cyclize 09/22/2009 Biochem: Carbohydrates II p. 17 of 58 b-Dglucopyranose Differs from aD-glucopyranose only at anomeric carbon 09/22/2009 Biochem: Carbohydrates II p. 18 of 58 Count carefully! It’s tempting to think that hexoses are pyranoses and pentoses are furanoses; But that’s not always true The ring always contains an oxygen, so even a pentose can form a pyranose In solution: pyranose, furanose, openchain forms are all present Percentages depend on the sugar 09/22/2009 Biochem: Carbohydrates II p. 19 of 58 Substituted monosaccharides Substitutions on the various positions retain some sugar-like character Some substituted monosaccharides are building blocks of polysaccharides Amination, acetylamination, carboxylation common O OOH HO HO O OH GlcNAc HNCOCH 3 09/22/2009 HO HO D-glucuronic acid HO (GlcUA) Biochem: Carbohydrates II O OH p. 20 of 58 6 Sugar acids (fig. 7.10) Gluconic acid: 5 4 1 3 D--gluconolactone 2 glucose carboxylated @ 1 position In equilibrium with lactone form Glucuronic acid: glucose carboxylated @ 6 position Glucaric acid: glucose carboxylated @ 1 and 6 positions Iduronic acid: idose carboxylated @ 6 09/22/2009 Biochem: Carbohydrates II p. 21 of 58 Sugar alcohols (fig.7.11) Mild reduction of sugars convert aldehyde moiety to alcohol Generates an additional asymmetric center in ketoses except dihyroxyacetone These remain in open-chain forms Smallest: glycerol Sorbitol, myo-inositol, ribitol are important 09/22/2009 Biochem: Carbohydrates II p. 22 of 58 Sugar esters (fig. 7.13) Phosphate esters of sugars are significant metabolic intermediates 5’ position on ribose is phosphorylated in nucleotides 09/22/2009 Biochem: Carbohydrates II Glucose 6phosphate p. 23 of 58 OH Amino sugars HO HO GlcNAc O OH HNCOCH3 Hydroxyl at 2- position of hexoses is replaced with an amine group Amine is often acetylated (CH3C=O) These aminated sugars are found in many polysaccharides and glycoproteins 09/22/2009 Biochem: Carbohydrates II p. 24 of 58 Hemiacetals and hemiketals Hemiacetals and hemiketals are compounds that have an –OH and an –OR group on the same carbon Cyclic monosaccharides are hemiacetals & hemiketals 09/22/2009 Biochem: Carbohydrates II p. 25 of 58 Acetals and ketals Acetals and ketals have two —OR groups on a single carbon Acetals and ketals are found in glycosidic bonds 09/22/2009 Biochem: Carbohydrates II p. 26 of 58 Oligosaccharides and other glycosides A glycoside is any compound in which the hydroxyl group of the anomeric carbon is replaced via condensation with an alcohol, an amine, or a thiol All oligosaccharides are glycosides, but so are a lot of monomeric sugar derivatives, like nucleosides 09/22/2009 Biochem: Carbohydrates II p. 27 of 58 Sucrose: a glycoside A disaccharide Linkage is between anomeric carbons of contributing monosaccharides, which are glucose and fructose 09/22/2009 Biochem: Carbohydrates II p. 28 of 58 Other disaccharides Maltose Cellobiose glc-glc with a-glycosidic bond from left-hand glc Produced in brewing, malted milk, etc. b-glc-glc Breakdown product from cellulose Lactose: b-gal-glc Milk sugar Lactose intolerance caused by absence of enzyme capable of hydrolyzing this glycoside 09/22/2009 Biochem: Carbohydrates II p. 29 of 58 Reducing sugars Sugars that can undergo ring-opening to form the open-chain aldehyde compounds that can be oxidized to carboxylic acids We describe those as reducing sugars because they can reduce metal ions or amino acids in the presence of base Benedict’s test: 2Cu2+ + RCH=O + 5OH- Cu2O + RCOO- + 3H2O Cuprous oxide is red and insoluble 09/22/2009 Biochem: Carbohydrates II p. 30 of 58 Ketoses are reducing sugars In presence of base a ketose can spontaneously rearrange to an aldose via an enediol intermediate, and then the aldose can be oxidized. 09/22/2009 Biochem: Carbohydrates II p. 31 of 58 Sucrose: not a reducing sugar Both anomeric carbons are involved in the glycosidic bond, so they can’t rearrange or open up, so it can’t be oxidized Bottom line: only sugars in which the anomeric carbon is free are reducing sugars 09/22/2009 Biochem: Carbohydrates II p. 32 of 58 Reducing & nonreducing ends Typically, oligo and polysaccharides have a reducing end and a nonreducing end Non-reducing end is the sugar moiety whose anomeric carbon is involved in the glycosidic bond Reducing end is sugar whose anomeric carbon is free to open up and oxidize Enzymatic lengthening and degradation of polysaccharides occurs at nonreducing end or ends 09/22/2009 Biochem: Carbohydrates II p. 33 of 58 Nucleosides Anomeric carbon of ribose (or deoxyribose) is linked to nitrogen of RNA (or DNA) base (A,C,G,T,U) Generally ribose is in furanose form This is an example of an N-glycoside 09/22/2009 Biochem: Carbohydrates II Diagram courtesy of World of Molecules p. 34 of 58 Polysaccharides Homoglycans: all building blocks same Heteroglycans: more than one kind of building block No equivalent of genetic code for carbohydrates, so long ones will be heterogeneous in length and branching, and maybe even in monomer identity 09/22/2009 Biochem: Carbohydrates II p. 35 of 58 Categories of polysaccharides Storage homoglycans (all Glc) Structural homoglycans Starch: amylose (a(14)Glc) , amylopectin Glycogen Cellulose (b(14)Glc) Chitin (b(14)GlcNAc) Heteroglycans Glycosaminoglycans (disacch.units) Hyaluronic acid (GlcUA,GlcNAc)(b(1 3,4)) 09/22/2009 Biochem: Carbohydrates II p. 36 of 58 Storage polysaccharides Available sources of glucose for energy and carbon Long-chain polymers of glucose Starch (amylose and amylopectin): in plants, it’s stored in 3-100 µm granules Glycogen Branches found in all but amylose 09/22/2009 Biochem: Carbohydrates II p. 37 of 58 Amylose Unbranched, a-14 linkages Typically 100-1000 residues Not soluble but can form hydrated micelles and may be helical Amylases hydrolyze a-14 linkages Diagram courtesy Langara College 09/22/2009 Biochem: Carbohydrates II p. 38 of 58 Amylopectin Mostly a-14 linkages; 4% a-16 Each sidechain has 15-25 glucose moieties a-16 linkages broken down by debranching enzymes 300-6000 total glucose units per amylopectin molecule One reducing end, many nonreducing ends 09/22/2009 Biochem: Carbohydrates II p. 39 of 58 Glycogen Principal storage form of glucose in human liver; some in muscle Branched (a-14 + a few a-16) More branches (~10%) Larger than starch: 50000 glucose One reducing end, many nonreducing ends Broken down to G-1-P units Built up from G-6-P G-1-P UDP-Glucose units 09/22/2009 Biochem: Carbohydrates II p. 40 of 58 Glycogen structure 09/22/2009 Biochem: Carbohydrates II p. 41 of 58 Structural polysaccharides I Insoluble compounds designed to provide strength and rigidity Cellulose: glucose b-14 linkages Rigid, flat structure: each glucose is upside down relative to its nearest neighbors (fig.7.27) 300-15000 glucose units Found in plant cell walls Resistant to most glucosidases Cellulases found in termites, ruminant gut bacteria Chitin: GlcNAc b-14 linkages: exoskeletons, cell walls (fig. 7.26) 09/22/2009 Biochem: Carbohydrates II p. 42 of 58 Structural polysaccharides II Alginates: poly(b-D-mannuronate), poly(a-L-guluronate), linked 14 Agarose: alternating D-gal, 3,6-anhydro-L-gal, with 6-methyl-D-gal side chains Cellulose-like structure when free Complexed to metal ions: 3-fold helix (“egg-carton”) Forms gels that hold huge amounts of H2O Can be processed to use in the lab for gel exclusion chromatography Glycosaminoglycans: see next section 09/22/2009 Biochem: Carbohydrates II p. 43 of 58 Glycoconjugates Poly or oligosaccharides covalently linked to proteins or peptides Generally heteroglycans Categories: Image courtesy Benzon Symposia Proteoglycans (protein+glycosaminoglycans) Peptidoglycans (peptide+polysaccharide) Glycoproteins (protein+oligosaccharide) 09/22/2009 Biochem: Carbohydrates II p. 44 of 58 Proteoglycans: Glycosaminoglycans Unbranched heteroglycans of repeating disaccharides One component is GalN, GlcN, GalNAc, or GlcNAc Other component: an alduronic acid —OH or —NH2 often sulfated Found in cartilage, joint fluid 09/22/2009 Biochem: Carbohydrates II p. 45 of 58 Proteoglycans in cartilage Highly hydrated, voluminous Mesh structure (fig.7.36 or this fig. from Mathews & Van Holde) Aggrecan is major proteoglycan Typical of proteoglycans in that it’s extracellular 09/22/2009 Biochem: Carbohydrates II p. 46 of 58 Peptidoglycans (G&G fig. 7.29) Polysaccharides linked to small proteins Featured in bacterial cell walls: alternating GlcNAc + MurNAc linked with b-(14) linkages Lysozyme hydrolyzes these polysaccharides Peptide is species-specific: often contains D-amino acids 09/22/2009 Biochem: Carbohydrates II p. 47 of 58 Peptidoglycans in bacteria Gram-negative: thin peptidoglycan layer separates two phospholipid bilayer membranes Gram-positive: only one bilayer, with thicker peptidoglycan cell wall outside it Gram stain binds to thick wall, not thin layer Fig. 7.30 shows multidimensionality of these walls 09/22/2009 Biochem: Carbohydrates II p. 48 of 58 Peptide component (G&G fig. 7.29) Sugars are crosslinked with entities containing (L-ala)-(isoglutamate)-(L-Lys)-(D-ala) Gram-neg: L-Lys crosslinks via D-ala Gram-pos: L-lys crosslinks via pentaglycine followed by D-ala 09/22/2009 Biochem: Carbohydrates II p. 49 of 58 Gram-negative bacteria: the periplasmic space (G&G fig. 7.30b, 7.31) Periplasmic space: space inside cell membrane but inside just-described peptidoglycan layer (note error in fig. legend!) Peptidoglycan is attached to outer membrane via 57-residue hydrophobic proteins Outer membrane has a set of lipopolysaccharides attached to it; these sway outward from the membrane 09/22/2009 Biochem: Carbohydrates II p. 50 of 58 Gram-negative membranes and periplasmic space QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Figure courtesy Kenyon College microbiology Wiki 09/22/2009 Biochem: Carbohydrates II p. 51 of 58 Glycoproteins 1-30 carbohydrate moieties per protein Proteins can be enzymes, hormones, structural proteins, transport proteins Microheterogeneity: same protein, different sugar combinations Eight sugars common in eukaryotes PTM glycosylation much more common in eukaryotes than prokaryotes 09/22/2009 Biochem: Carbohydrates II p. 52 of 58 Diversity in glycoproteins Variety of sugar monomers a or b glycosidic linkages Linkages always at C-1 on one sugar but can be C-2,3,4,6 on the other one Up to 4 branches But: not all the specific glycosyltransferases you would need to get all this diversity exist in any one organism 09/22/2009 Biochem: Carbohydrates II p. 53 of 58 O-linked and Nlinked oligosaccharides Characteristic sugar moieties and attachment chemistries 09/22/2009 Biochem: Carbohydrates II p. 54 of 58 O-linked oligosaccharides (fig. fig 7.32a, 7.33 in G&G) GalNAc to ser or thr; often with Gal or Sialic acid on GalNAc 5-hydroxylysines on collagen are joined to D-Gal Some proteoglycans joined via Gal-Gal-Xyl-ser Single GlcNAc on ser or thr 09/22/2009 Biochem: Carbohydrates II p. 55 of 58 N-linked oligosaccharides (fig. 7.32b,c in G&G) Generally linked to Asn Types: High-mannose Complex (Sialic acid, …) Hybrid (Gal, GalNAc, Man) 09/22/2009 Biochem: Carbohydrates II Diagram courtesy Oregon State U. p. 56 of 58 iClicker question 4 Suppose you isolate a polysaccharide with 5000 glucose units, and 3% of the linkages are 1,6 crosslinks. This is: (a) amylose (b) amylopectin (c) glycogen (d) chitin (e) none of the above. 09/22/2009 Biochem: Carbohydrates II p. 57 of 58 iClicker question 5 Suppose you isolate an enzyme that breaks down b-1,4-glycosidic linkages between GlcNAc units. This would act upon: (a) glycogen (b) cellulose (c) chitin (d) all of the above (e) none of the above. 09/22/2009 Biochem: Carbohydrates II p. 58 of 58