* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download heme
Peptide synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Human digestive system wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Citric acid cycle wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Biochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Biosynthesis of doxorubicin wikipedia , lookup
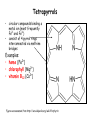
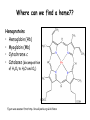
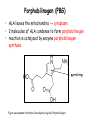
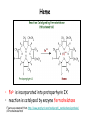
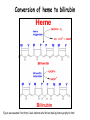
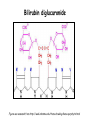
Metabolism of tetrapyrrols Pavla Balínová Tetrapyrrols • circular compounds binding a metal ion (most frequently Fe2+ and Fe3+) • consist of 4 pyrrol rings interconnected via methine bridges Examples: • heme (Fe2+) • chlorophyll (Mg2+) • vitamin B12 (Co2+) Figure was assumed from http://en.wikipedia.org/wiki/Porphyrin Where can we find a heme?? Hemoproteins • Hemoglobin (Hb) • Myoglobin (Mb) • Cytochrome c • Catalases (decomposition of H2O2 to H2O and O2) Figure was assumed from http://en.wikipedia.org/wiki/Heme Heme structure methine bridge pyrrol Figure was assumed from a book T. M. Devlin et al.: Textbook of Biochemistry With Clinical Correlations, 4th ed., Wiley-Liss, Inc., New York, 1997. Biosynthesis of heme • Organ location: bone marrow 85% and liver • Subcellular location: mitochondria and cytosol • Substrates: succinyl-CoA and glycine • Important intermediates: δ-aminolevulinic acid (ALA), porphobilinogen, uroporphyrinogen III, protoporphyrin IX • Key regulatory enzyme: ALA synthase Heme biosynthesis Figure was assumed from http://www.porphyrin.net/mediporph/_netbiochem/synthesis/_synthmain.html Delta-aminolevulinic acid (ALA) • synthesis of heme starts in mitochondria • succinyl-CoA and glycine (Gly) undergo condensation → δ-aminolevulinic acid (ALA) • reaction is catalyzed by enzyme ALA synthase -OOC-CH 2-CH2-CO-S-CoA + NH3+-CH2-COO- CO2 -OOC-CH -CH -CO-CH -NH + 2 2 2 3 Porphobilinogen (PBG) • ALA leaves the mitochondria → cytoplasm • 2 molecules of ALA condense to form porphobilinogen • reaction is catalyzed by enzyme porphobilinogen synthase pyrrol ring Figure was assumed from http://en.wikipedia.org/wiki/Porphobilinogen Uroporphyrinogen III Figure was assumed from book T. M. Devlin et al.: Textbook of Biochemistry With Clinical Correlations, 4th ed., Wiley-Liss, Inc., New York, 1997. Uroporphyrinogen → coproporphyrinogen III • enzyme hydroxymethylbilane synthase catalyzes the linkage of 4 PBG molecules and cleavage of 4 NH4+ to yield uroporphyrinogen III • 4 acetate residues are decarboxylated into methyl groups → coproporphyrinogen III returns to the mitochondria again Protoporphyrinogen IX Figure was assumed from book T. M. Devlin et al.: Textbook of Biochemistry With Clinical Correlations, 4th ed., Wiley-Liss, Inc., New York, 1997. Protoporphyrinogen IX → protoporphyrin IX • oxidation of protoporphyrinogen IX produces the conjugated π-electron system of protoporphyrin IX Figure was assumed from book T. M. Devlin et al.: Textbook of Biochemistry With Clinical Correlations, 4th ed., Wiley-Liss, Inc., New York, 1997. Heme • Fe2+ is incorporated into protoporhyrin IX • reaction is catalyzed by enzyme ferrochelatase Figure was assumed from http://www.porphyrin.net/mediporph/_netbiochem/synthesis/ ferrochelatase.html Regulation of heme biosynthesis ALA synthase is a key regulatory enzyme • it is an allosteric enzyme that is inhibited by heme = feedback inhibition • requires pyridoxal phosphate • certain drugs and steroid hormones can increase heme synthesis Porphobilinogen synthase is inhibited by lead ions Pb2+ in case of lead poisoning. Ferrochelatase can be also inhibited by Pb2+. Its activity is influenced by availability of Fe2+ and ascorbic acid. Porphyrias • are hereditary or acquired disturbances of heme synthesis • in all cases there is an identifiable abnormality of the enzymes which synthesize heme • this leads to accumulation of intermediates of the pathway and a deficiency of heme → excretion of heme precursors in feces or urine, giving them a dark red color ● accumulation of porphyrinogens in the skin can lead to photosensitivity • the neurological symptoms Heme degradation • around 100 – 200 million aged erythrocytes per hour are broken down in the human organism • Organ location: RES (reticuloendothelial cells) in the spleen, liver and bone marrow Hb is degraded to: ● globin → AAs → metabolism ● heme → bilirubin 2+ → transport with transferrin and used in the next ● Fe heme biosynthesis Not only Hb but other hemoproteins also contain heme groups which are degraded by the same pathway. Conversion of heme to bilirubin Figure was assumed from http://web.indstate.edu/thcme/mwking/heme-porphyrin.html Bilirubin Bilirubin (Bil) is released from RES into the blood. BUT! Bil is only poorly soluble in plasma, and therefore during transport it is bound to albumin. ↓ LIVER In the hepatocytes, Bil is conjugated by 2 molecules of glucuronic acid → bilirubin diglucuronide (soluble in water, „conjugated Bil“). Conjugation is catalyzed by UDP-glucuronosyltransferase. Bilirubin diglucuronide Figure was assumed from http://web.indstate.edu/thcme/mwking/heme-porphyrin.html Bile pigments bilirubin diglucuronide ↓ BILE ↓ INTESTINE Bil is reduced to urobilinogen and stercobilinogen by bacteria → oxidation to urobilin and stercobilin Bile pigments are mostly excreted in feces, but a small proportion is resorbed (enterohepatic circulation). Small amount of urobilinogen is excreted with urine. Determination of bilirubin in serum Blood tests • Bil reacts directly when dyes are added to the blood sample → conjugated bilirubin = direct • free Bil does not react to the reagents until alcohol (methanol) or caffeine is added to the solution. Therefore, the measurement of this type of bilirubin is indirect → unconjugated bilirubin = indirect • Total bilirubin measures both unconjugated and conjugated Bil (normal value up to 20 µmol/L). Hyperbilirubinemias • Hyperbilirubinemia = an elevated bilirubin level (> 10 mg/L) → Bil can diffuses from the blood into peripheral tissues and gives it a yellow color (jaundice = icterus) Jaundice can have various causes: • Increased erythrocyte degradation – hemolytic jaundice • Impaired conjugation of bilirubin in the liver – hepatocellular jaundice • Disturbance of bile drainage (gallstones) – obstructive jaundice In the urine, only conjugated bilirubin can be present. Icterus Icterus is the yellow coloration of skin and mucus membranes of jaundice (hyperbilirubinemia with various ethiology) • Hemolytic icterus: elevated level of unconjugated Bil in blood • Neonatal jaundice usually appears after a few days after birth (elevated hemolysis, decreased activity of UDP-glucuronosyltransferase → ↑ unconjugated Bil) In severe cases, unconjugated Bil can cross the bloodbrain barrier and lead to brain damage (kernicterus).