* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Macromolecules_students
Light-dependent reactions wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Metalloprotein wikipedia , lookup
Protein structure prediction wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
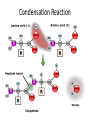
Matter Cycles ; Energy Flows Bio300 Mrs. Lil Macromolecule Chart Use your brains, the books, the notes, & your classmates’ rows to fill in… MONOMER LIPIDS CARBOHYDRATES NUCLEIC ACID (DNA/RNA) PROTEIN STRUCTURE NOTES • We eat food to get the matter & the energy! What do they all have in common? 6 most common atoms for life Main Ideas • Today we reviewed atomic structure & the four most important macromolecules of life • Electrons are responsible for what we call “energy” in biology. – a BOND is the sharing or attraction of electrons between two atoms – Life is carbon-based b/c its four ‘sharable’ valence e- allow it to bond in all directions • Summarize why today’s topic was: “matter cycles” Keystone Content Today • Compare the structure and function of carbohydrates, lipids, proteins, and nucleic acids in organisms • Explain how carbon is uniquely suited to form biological macromolecule Matter Cycles ; Energy Flows Bio300 Mrs. Lil Seating Challenge #4 • Can you make a food chain? • Sit at a table group where everyone’s tag is a different color. –Limit 3-4 people per group –Please get your syllabus out too so I can check the signature • http://www.npr.org/2007/05/01/9943298/epi sode-1-its-all-about-carbon • Episode 2 (3min) Food Chain • Yesterday you snacked on food. • What was it that you ate exactly? • How is it part of a food chain? • Arrange the organisms (necklackes) on your desk into a correct food chain & copy the work in you notebook • What do the arrows represent? • • • • Carnivore Decomposer Producers Herbivore Stand up if you’re… True or False? If you ate 20 pounds of food, you would gain 20 pounds The Popcorn Race… 1. The popcorn represented….. 2. When did the popcorn spill? 3. What would happen if there were fewer transfers? What do you think this has to do with food chains? • Why are food chains rarely more than 4 or 5 members (trophic levels) long? How many trophic levels can there be in a chain? Heat First Trophic Level Second Trophic Level Third Trophic Level Fourth Trophic Level Producers (plants) Primary consumers (herbivores) Secondary consumers (carnivores) Tertiary consumers (top carnivores) Heat Heat Heat Solar energy Heat Heat Detritvores (decomposers and detritus feeders) Heat Matter Cycles – Energy Flows With your table, Build a molecule! • We eat food to get the matter & the energy! 9 / 9 Do Now: • In your notebook…. 1) Of the three macromolecules : lipid, carb, protein – hypothesize which one you think holds the MOST energy & will therefore change the temp of water most. - hint : check your chart notes 2) What is the preferred method to extinguish a lab fire? 3) Get out your lab & review the procedure So….Was your hypothesis supported? • If these were two of the foods you were trying to compare, what information would you look for on the label to understand how much energy is stored? • Get out lab & notebook • http://www.npr.org/2007/05/01/9943298/epi sode-1-its-all-about-carbon • Episode 3 (3min) Can you identify which macromolecule? If bonds hold energy, what molecule has the most energy? Get out your macromolecule chart please Saturated vs. Unsaturated • • • • All single bonds connect C Solid at room temp Ex: butter, lard “Straight, stackable” • • • • Contain double bonds Liquid at room temp Ex: olive oil, corn oil Typically plant-based What are trans-fats? • “Trans” double bonds are not naturally found in biological systems • When unsat. fats are “hydrogenated” to become sat. fat (easier to store, ship,use), the H’s can rearrange and ‘straighten out’ the molecule • Trans fat is bad (?) b/c it is not recognized by our body’s enzymes (?) • Answer the conclusion questions in your lab packet. • What was the main point? Do Now: Question Analysis 1) Decide with your tablemates what the best answer is. 2) Be ready to explain why one of the letters would be wrong The enzyme amylase will affect the breakdown of carbohydrates, but it will not affect the breakdown of proteins. The ability of an enzyme molecule to interact with specific molecules is most directly determined by the: A. shapes of the molecules involved B. sequence of bases present in ATP C. number of molecules involved D. amount of glucose present in the cell We don’t have fire in our stomach! • So how do we digest food? – Aka – get the energy out of the bonds? ________ – Get your macromolecule chart out; flip over. Proteins • Check your macromolecule chart • Fxn: control reactions (enzymes), regulate cell processes, structure (tissues, bones, muscles), transport & help fight disease • Structure: contain N, C, H, O – Have an amino group (-NH2) – Have a carboxyl group (-COOH) – Have an “R” group (“other”) • there are 20 different R groups • Three major groups: Polar, Ionic, and Nonpolar It’s all about shape… • 1st objective today – how does a protein get its shape? – Amino acid + Amino acid = Protein – Monomer + Monomer = Polymer • 2nd objective – What happens when that shape is ruined? – Aka Denatured Polymerization Synthesis of organic molecules Small subunits called MONOMERS are joined to form POLYMERS Polymers are MACROMOLECULES Monomers Polymer Types of Reactions • Hydrolysis – Break apart monomers – by adding water. – An H is added to one monomer & an OH is added to the other monomer. • Dehydration Synthesis ( or Condensation) – – – – Join monomers One monomer loses a H+ and the other loses an OHWater is removed Covalent bond is formed Condensation Reaction Keystone Check The diagram shows a reaction that forms a polymer from two monomers. What is this type of reaction called? a. glycolysis c. photosynthesis b. hydrolysis d. dehydration synthesis Activity! • We’re going to become a “class polypeptide” 1) Hold the “carboxyl group” in your right hand 2) Hold the “amine group” in your left hand 3) Your head is the “H” 4) Your legs are all different, so they’re the “R” group – meaning the “other” group 20 Possible R groups (red) Main Idea: each amino acid is unique with its own properties. Like stays with Like. When in a long chain, polypeptides (aka protein) folds uniquely Putting it together…. The contents of the small intestine have a basic pH. When gastric protease enters the small intestine, the activity of the enzyme will most likely : A. increase, only B. decrease, only C. increase and then decrease D. remain the same Everyday examples • What happens with heat? Acid? • Ex 1 - Substrate = Eggs • Ex 2 - Substrate = Apples How about this one? A scientist observes that, when the pH of the environment surrounding an enzyme is changed, the rate the enzyme catalyzes a reaction greatly decreases. Which statement best describes how a change in pH can affect an enzyme? A. A pH change can cause the enzyme to change its shape. B. A pH change can remove energy necessary to activate the enzyme. C. A pH change can add new molecules to the structure of the enzyme. D. A pH change can cause an enzyme to react with a different substrate.