* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Redox reaction during glycolysis
Survey
Document related concepts
Mitochondrion wikipedia , lookup
Phosphorylation wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Metalloprotein wikipedia , lookup
Photosynthesis wikipedia , lookup
Electron transport chain wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Microbial metabolism wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biochemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
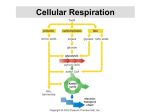
CELLULAR RESPIRATION TOPIC 3.7 (core) and TOPIC 8.1 (HL) Topic 3.7 Cellular respiration Objectives • Define cell respiration. • State that, in cell respiration, glucose in the cytoplasm is broken down by glycolysis into pyruvate, with a small yield of ATP. • Explain that, during anaerobic cell respiration, pyruvate can be converted in the cytoplasm into lactate, or ethanol and carbon dioxide, with no further yield of ATP. • Explain that, during aerobic cell respiration, pyruvate can be broken down in the mitochondrion into carbon dioxide and water with a large yield of ATP. • Which form of energy is used by living things? Living things use chemical energy for their biological work. • What is the main source of energy for the Earth? SUN • How do living things get this energy? What are the biological works which require energy? - Synthesis of new biomolecules Active transport Cell division Movement • Energy can be converted from one form to another, but it is neither created nor destroyed. • SUN plants ATP photosynthesis organic molecules cellular activities * All cells use ATP for their cellular activities. • • • • Organic molecules have chemical energy. 1gr carbohydrate 4.2 kcal 1gr protein 4.3 kcal 1gr lipid 9.3 kcal • Why do lipids store more energy than other organic molecules? Structure of ATP ATP PRODUCTION 1- Substrate level phosphorylation: is the production of ATP by the direct transfer and donation of phosphoryll group to ADP from a phosphorylated substrate. 2- Oxidaditive phoshorylation: production of ATP by redox reaction in the presence of oxygen 3- Photophosphorylation: production of ATP by light energy. 4- Chemophosphorylation: production of ATP by oxidation of inorganic substances such as NO3, Fe2+ GENERAL FORMULA OF CELLULAR RESPIRATION glycolysis Glucose Pyruvic acid Aerobic respiration in mitochondria fermentation Ethanol İn yeast Lactic acid in muscle cell GLYCOLYSIS • Series of reactions which are common in aerobic reactions and anaerobic reactions. (all living cells do glycolytic reactions) • Take place in cytoplasm. • During glycolysis 2 ATP are used and 4 ATP, 2 NADH2 (coenzyme), 2 H2O and 2 pyruvic acids are produced. • ATP are produced by substrate level phosphorylation. • At the end of the glycolysis 2 ATP are gained. CITRIC ACID (KREBS) CYCLE ELECTRON TRANSPORT CHAIN • ETS is a group of protein that transfer electrons. • Most of the ATPs are produced by oxidative phoshorylation in the ETS. LACTIC ACID FERMENTATION ETHYL ALCOHOL FERMENTATION HL TOPIC 8.1:Cell respiration • • • • • • State that oxidation involves the loss of electrons from an element, whereas reduction involves a gain of electrons; and that oxidation frequently involves gaining oxygen or losing hydrogen, whereas reduction frequently involves losing oxygen or gaining hydrogen. Outline the process of glycolysis, including phosphorylation, lysis, oxidation and ATP formation. Draw and label a diagram showing the structure of a mitochondrion as seen in electron micrographs. Explain aerobic respiration, including the link reaction, the Krebs cycle, the role of NADH + H+, the electron transport chain and the role of oxygen. Explain oxidative phosphorylation in terms of chemiosmosis. Explain the relationship between the structure of the mitochondrion and its function. • Cellular respiration is an oxidation reduction (redox) rection. HL-8.1.1 REDOX REACTIONS IN CELLULAR RESPİRATİON * In the living things oxidation means; H atoms leave organic molecules. At the same time H atoms take energy and electrons from the chemical bond between H and C atom of the organic compound. *These H atoms bind with coenzymes (NAD and FAD). That means e and energy are transferred to coenzymes. *NAD and FAD transfer e, H and energy to ETS which are used for oxidative phosphorylation. • In the areobic and anaerobic reactions organic molecules (glucose) are oxidized (give electrons). • Which molecules are reduced? *In arebic respiration during glycolysis and Krebs cycle e are taken from glucose and transferred in the ETS. The last e acceptor is oxygen. *In fermentation last e acceptor is pyruvic acid and acetaldehyde. Redox reaction during glycolysis Glucose 2 ATP 3C molec 3C molec 2H 2H 2 ATP 2 ATP pyruvate pyruvate 2H 2H Acetyle Co-A Acetyl-Co A Krebs cycle 2H Krebs cycle 2H 2H 2H 2H 2H 2H 2H ETS Electron transport system (ETS) • Chemiosmosis Chemiosmosis • NADH+H+ supplies pair of H atoms to the first carrier in the chain, with the NAD+ returning to the matrix. • The hydrogen atoms are split, to release two electrons, which pass from carrier in the chain. • Energy is released as the e- pass from carrier to carrier, and three of these use this energy to transfer protons (H+ ) across the inner membrane space. • As electrons continue to flow along the chain and more and more protons are pumped across the inner mitochondrial space, a concentration of protons builds up. The proton gradient is the store for potential energy. • To allow e- to continue to flow, they must be transferred to a terminal e acceptor at the end of the chain. In aerobic respiration it is Oxygen (02 ). When combines with hydrogen it forms water. • Protons pass back to matrix through ATP synthase. As they move down the concentration gradient, energy is released and this energy is used to make ATP.