* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1 Types of Chemical Reactions
Chemistry: A Volatile History wikipedia , lookup
Green chemistry wikipedia , lookup
Synthesis of carbon nanotubes wikipedia , lookup
Chemical potential wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Organic chemistry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Isotopic labeling wikipedia , lookup
Drug discovery wikipedia , lookup
Chemical plant wikipedia , lookup
History of molecular theory wikipedia , lookup
Safety data sheet wikipedia , lookup
Chemical weapon wikipedia , lookup
Fluorochemical industry wikipedia , lookup
Chemical industry wikipedia , lookup
Water pollution wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Process chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Chemical Corps wikipedia , lookup
Biosequestration wikipedia , lookup
Electrochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Abiogenesis wikipedia , lookup
Total organic carbon wikipedia , lookup
Transition state theory wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Water splitting wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Click chemistry wikipedia , lookup
History of chemistry wikipedia , lookup
Atomic theory wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Chemical reaction wikipedia , lookup
Photosynthesis wikipedia , lookup
Electrolysis of water wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Biochemistry wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
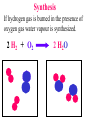
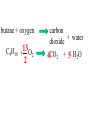
Since atoms and molecules are too small to see chemical changes are detected by the following observations: A colour change occurs. A new gas with different properties is formed. Sudden dramatic changes in temperature occur. A new solid suddenly appears. Chemical Changes are represented by word equations and chemical equations. When propane gas burns in air it produces carbon dioxide and water. The word equation for this reaction is: Reactants Products propane + oxygen => carbon dioxide + water are consumed are produced The chemical equation is: C3H8 + 5 O2 -----> 3 CO2 + 4 H2O The balanced equation is: Types of Chemical Reactions During chemical changes new substances with different physical and chemical properties are formed. Chemical reactions can be grouped into categories depending on the nature of the chemical reaction. For example during a decomposition reaction a single substance can break apart into several different substances. Decomposition Notice the intact particle on the left breaks apart into multiple particles on the right. Decomposition Potassium chlorate can be made to decompose using heat. When it decomposes it forms potassium chloride and oxygen gas. 2 KClO3 2 KCl 3 O2 Single Displacement This type of reaction is similar to a single person at a dance “butting” in and stealing someone’s partner. Single Displacement If copper metal is placed in a solution of silver nitrate the silver will replace the copper. Cu + 2AgNO3 2Ag Cu(NO3)2 See chapter 4, lesson 16 , Saunders Interactive Chemistry Double Displacement guy girl guy girl This type of reaction is similar to a partner “swap”. Remember only guy - girl pairings. Double Displacement If potassium iodide is placed in a solution of lead(II) nitrate the products are Potassium nitrate and lead(II) iodide PbI2 2 KNO3 2 KI + Pb(NO3)2 See chapter 4, lesson 16 , Saunders Interactive Chemistry Synthesis When 2 or more substances combine to form a substance with larger molecules it is called a synthesis. Proteins are synthesized from 20 different amino acids. Synthesis If hydrogen gas is burned in the presence of oxygen gas water vapour is synthesized. 2 H2 + O2 2 H2O Combustion Reactions occur when fuels are combined with oxygen. The products are water vapour and carbon dioxide if it is a complete combustion. Incomplete combustions produce carbon monoxide and water and/or carbon and water. Here is an example of a complete combustion: butane + oxygen C4H10 +13O2 2 carbon + water dioxide 4CO2 + 5 H2O