* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Diapositive 1
Protein structure prediction wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Protein purification wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein domain wikipedia , lookup
Western blot wikipedia , lookup
Trimeric autotransporter adhesin wikipedia , lookup
Protein–protein interaction wikipedia , lookup
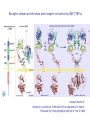
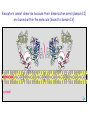
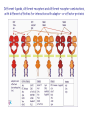
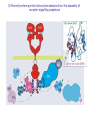
The Ras – MAP kinase pathway I The big family of Receptor Tyrosine Kinases Receptor domain architecture and receptor activation by EGF (TGFa) unusual mode of allosteric activation, formation of an asymmetric dimer, followed by trans-phosphorylation of the C-tails Receptors cannot dimerize because their dimerization arms (domain-II) are buried within the molecule (bound to domain IV) II membrane cytosol II The dimerized receptor; interaction between II-domains (« dimerization arms ») II membrane II Detail of the transphosphorylation reaction of Cterminal tails by dimerized EGF receptors ATP tyrosine Phophorylated tyrosines and their surrounding amino acids form docking sites for adaptor- and effector proteins Different ligands, different receptors and different receptor-combinations, with different affinities for interaction with adaptor- or effector proteins A) When large scale micro-array protein interaction protocoles are applied, « the tough gets going ». This picture illustrates the « interactome » of different phosphorylated EGF receptor C-terminal tails B) Note that with ERBB2, interactions vary greatly with increasing concentrations of cytoplasmic tails (i.e. overexpression of ERBB2 may change signalling patterns). Image from: Jones et al. Nature 2006;439:168174) Domain architecture of adaptor-, docking- and effector proteins Different protein-protein interaction domains drive the assembly of receptor signalling complexes Branching of signal transduction pathway (signalling network) Sos mediates guanine nucleotide exchange by widening the nucleotide binding pocket and by hindering Ser 17 (inside the circle) to interact with GDP. Ras GDP glu942 hSos Activation of Ras means; rearranging the switch regions through the tight link between threonine 35 (in pink) and the third (g)phosphate of ATP. This rearrangement creates favourable interaction sites with effectors Ras inactive GTP GDP Ras GDP Ras active Effector loop; no interaction Ras GTP interaction with Raf-RBD: effector loop Raf binds to the Ras effector loop (aa 32-40) with its Ras-binding domain (RBD). The image below shows how the switch-II region of RasGDP hinders the interaction with Raf effector loop Ras Ras GTP GDP Raf-RBD Switch II region switch regions RasGTP induces a cascade of phosphorylation reactions, in which each kinase activates the other by phosphorylation of residues in the activation segment. ERK2 dimerizes and enters the nucleus Exactly how Raf is activated and how ERK2 enters the nucleus remains uncertain MEK activates MAPkinase by phosphorylation of Thr183 and Tyr185. MEK is a rather unusual protein kinase because of its highly restricted choice of substrates MAPKinase inactive MAPKinase active ATP Pthr183 thr183 Ptyr185 tyr185 Activation segment Substrate entry site Activated ERKK2 enters the nucleus and phosphorylates transcription factors. In the case of Elk-1 this leads to dimerization with SRF whereas in the case of c-Fos this results in a prolonged half-life and thus more effective induction of transcription. With respect to the cell division cycle, expression of cyclinD is one of the consequences of the action of ERK2. ERK2 is a member of a large family of mitogen-activated protein kinases (MAPK) which share sequence identity as well as mode of activation ERK2, as well as other members of the MAPK family, phosphorylates and activates yet other protein kinases. These too constitute a family, the MAPKactivated protein kinases. MNK1, MAP-kinase interacting kinase, is phosphorylated and activated by ERK2. It binds the eukaryotic initiation factor eIF-4E and eIF-4G. MNK1 phosphorylates eIF4E and this facilitates the association of eIF-3, an essential protein for the assembly of the ribosomal complex One of the genes induced by the MAPkinase pathway is the dual specificity phosphatase MKP-1. It dephosphorylates and de-activates MAPK, both in the nucleus and the cytoplasm, on its thr-tyr residues The Ras-ERK signal transduction pathway in other species Ras-ERK and development of the ommatidium in Drosophila (rhabdomere formation) Ras-ERK and the development of the vulva in Caenorhabdites elegans