* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Proteins & Nucleic Acids - St. Mary Catholic Secondary School
Survey
Document related concepts
Bimolecular fluorescence complementation wikipedia , lookup
Structural alignment wikipedia , lookup
Homology modeling wikipedia , lookup
Protein domain wikipedia , lookup
Protein purification wikipedia , lookup
Protein folding wikipedia , lookup
RNA-binding protein wikipedia , lookup
Protein moonlighting wikipedia , lookup
Circular dichroism wikipedia , lookup
Western blot wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
List of types of proteins wikipedia , lookup
Transcript
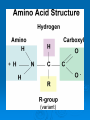
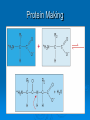
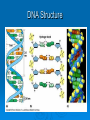
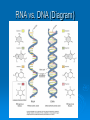
Proteins & Nucleic Acids Also Known As… The Workers & Directors Proteins Also Known As… The “Rock Star” Worker Molecules Protein – Basic Info Proteins are the “working” molecules of the body. They can act as enzymes, aiding in chemical reactions, or be structural components such as fingernails. Proteins have a very specific 3-D shape which relates directly with their function – if this shape is not exact in every way, the protein may not function at all. On top of this, if the conditions in which the proteins must function are not just right – the protein may function at a lower capacity or not at all – even if it had the right shape to start. Think of proteins as the spoiled rock stars of the body – if they are not feeling right or the stage or dressing room they have to perform in is a little off – the show may be cancelled altogether. Protein Building Blocks The monomer for the proteins is the amino acid. There are 20 different amino acids. They have the same “body” with a different “head”. The “body” of an amino acid, that is common to all 20 of them, has the following parts: An alpha (α)carbon – the central carbon that holds it all together. There is always a hydrogen bonded to this alpha carbon. An amino group – NH2. A carboxyl group – COOH. There is also an R-Group – this is the head of the amino acid that is used to identify which of the 20 amino acids it happens to be. Making Proteins Amino acids are bonded together using a dehydration synthesis reaction – remove the water and connect the leftovers. The water comes from a “H” on the amino group and the “OH” of the carboxyl group. The bond that is made between the amino acids is called a peptide bond. This means, of course, that proteins are taken apart by hydrolysis – stick the water back in and break it up. This is what you do when you eat a hamburger and break down the protein of the beef. Protein Making Protein Structure (Shape) Structural proteins tend to be linear. The proteins that act as enzymes have a more pronounced 3-D that is absolutely necessary for the proper function of the enzyme – damn rock stars! There are 4 levels of protein structure… Primary – straight line. Secondary – α-helix or β-pleated sheet. Tertiary – “Kinky slinky” with folds. Quaternary – A bunch of tertiary proteins acting as one. Primary & Secondary Structure Primary structure is the straight chain of amino acids that has just been built. This structure can be called a polypeptide – many peptide bonds. aa-aa-aa-aa-aa-aa-aa-aa-aa-aa-aa-etc… Secondary structure sees this chain assume the α-helix (corkscrew) shape or a β-pleated sheet (fan) shape. The secondary structure is held together by hydrogen bonding between nearby carboxyl and amino groups within the polypeptide chain. Tertiary & Quaternary Structure Tertiary structure occurs when the helix and sheet interact and twist around each other – it’s like a slinky and a fan getting mangled together. Tertiary shape is held together by R-group bonding within the chain and R-group interactions with the environment. Tertiary structure is also aided by prosthetic groups that are inorganic compounds that act as a central point for bonding within the protein. Quaternary structure occurs when a few tertiary structures fit together to act as one functional unit. Go Exact Or Go Home!!! Proteins are the rock stars of the body but they are very specific in their construction – if they are not built exactly right – they may not work. This need for the exact 3-D shape is known as specificity and it is based on two things: The number of amino acids. The order of the amino acids. If the protein is built exactly right it will function properly unless environmental conditions get to tough. I Fall To Pieces… If the environment is too tough – the protein may denature – fall apart and lose that special 3-D shape. Denaturing may occur is response to: Temperature changes – especially heat. Changes in pH. Changes in salt/ion concentration. In your body, a protein may denature but chaperone proteins will help reassume the proper shape. In a lab, there are no chaperone proteins so once the protein denatures – it is gone for good! Nucleic Acids Also Known As… The Directions on How to Make Proteins Just Right. Nucleic Acids – Basic Info They carry genetic information. This information determines all of your structural and functional characteristics. DNA – Deoxyribonucleic Acid - houses the genetic code within the nucleus of the cell. RNA – Ribonucleic Acid - carries a copy of the code to the protein-making areas of the cell in the cytoplasm. ATP – the cell’s energy – is also made from a nucleic acid building block. Building DNA & RNA The monomer for the nucleic acids is the nucleotide. The nucleotide has three parts: 5-carbon Sugar Phosphate group Nitrogen-containing Base It‘s the sequence of the nitrogenous bases that provides the code/instructions for making the proteins. DNA Double helix composed of two strands of nucleotides held together by hydrogen bonds. The two strands are antiparallel to each other and are made of millions of nucleotides. Ladder shape – Rails - A series of alternating phosphates and sugars linked by covalent bonds known as phosphodiester bonds. Rungs of the ladder are made of the nitrogenous bases and their hydrogen bonds. The nitrogenous bases involved with DNA are adenine, cytosine, guanine and thymine. The adenine and thymine pair up using two hydrogen bonds and cytosine and guanine pair up using three hydrogen bonds (Chargaff’s Base-Pair Rule). The nitrogenous bases can be either purines (A & G) or pyrimidines (C & T or U in RNA). DNA Structure RNA RNA is a code-carrying, single-stranded molecule made of many nucleotides. There are several differences between RNA and DNA. RNA vs. DNA DNA Double-stranded. A, C, G & T. Deoxyribose - sugar. H-bonds present. Works in nucleus. RNA Single-stranded. A, C, G, & U. Ribose - sugar. No H-bonds Works in cytoplasm – built in nucleus. RNA vs. DNA (Diagram) ATP ATP (Adenosine Triphosphate) is the cellular form of energy. It is composed of three phosphates, a ribose sugar and an adenine base. The energy of the molecule is found in the bonds between the phosphate groups. ATP Structure FIN!