* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Enzyme Mechanisms - Illinois Institute of Technology
Gaseous signaling molecules wikipedia , lookup
Proteolysis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
Western blot wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Restriction enzyme wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Metalloprotein wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Biosynthesis wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Biochemistry wikipedia , lookup
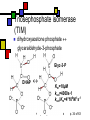
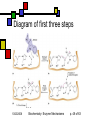
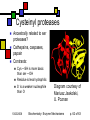
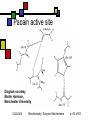
Enzyme Mechanisms Andy Howard Introductory Biochemistry, Fall 2008 Thursday 23 October 2008 Biochemistry: Enzyme Mechanisms 1 10/23/2008 How do enzymes reduce activation energies? We want to understand what is really happening chemically when an enzyme does its job. We’d also like to know how biochemists probe these systems. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 2 of 63 Mechanism Topics Inhibitors, concluded: Pharmaceuticals Mechanisms: Terminology Transition States Enzyme chemistry 10/23/2008 Diffusion-controlled Reactions Binding Modes of Catalysis Induced-fit Tight Binding of Ionic Intermediates Serine proteases Biochemistry: Enzyme Mechanisms p. 3 of 63 Most pharmaceuticals are enzyme inhibitors Some are inhibitors of enzymes that are necessary for functioning of pathogens Others are inhibitors of some protein whose inappropriate expression in a human causes a disease. Others are targeted at enzymes that are produced more energetically by tumors than they are by normal tissues. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 4 of 63 Characteristics of Pharmaceutical Inhibitors Usually competitive, i.e. they raise Km without affecting Vmax Some are mixed, i.e. Km up, Vmax down Iterative design work will decrease Ki from millimolar down to nanomolar Sometimes design work is purely blind HTS; other times, it’s structure-based 10/23/2008 Biochemistry: Enzyme Mechanisms p. 5 of 63 Amprenavir Competitive inhibitor of HIV protease, Ki = 0.6 nM for HIV-1 No longer sold: mutual interference with rifabutin, which is an antibiotic used against a common HIV secondary bacterial infection, Mycobacterium avium 10/23/2008 Quic kTime™ and a TIFF (Unc ompres sed) decompress or are needed to see this picture. Biochemistry: Enzyme Mechanisms p. 6 of 63 When is a good inhibitor a good drug? It needs to be bioavailable and nontoxic Beautiful 20nM inhibitor is often neither Modest sacrifices of Ki in improving bioavailability and non-toxicity are okay if Ki is low enough when you start sacrificing 10/23/2008 Biochemistry: Enzyme Mechanisms p. 7 of 63 How do we lessen toxicity and improve bioavailability? Increase solubility… that often increases Ki because the van der Waals interactions diminish Solubility makes it easier to get the compound to travel through the bloodstream Toxicity is often associated with fat storage, which is more likely with insoluble compounds 10/23/2008 Biochemistry: Enzyme Mechanisms p. 8 of 63 Drug-design timeline 2 years of research, 8 years of trials -8 Research 0 10/23/2008 2 100 Cost/yr, 106 $ -3 Preliminary toxicity testing 10 Clinical Trials Time, Yrs Biochemistry: Enzyme Mechanisms 10 p. 9 of 63 Atomic-Level Mechanisms We want to understand atomic-level events during an enzymatically catalyzed reaction. Sometimes we want to find a way to inhibit an enzyme in other cases we're looking for more fundamental knowledge, viz. the ways that biological organisms employ chemistry and how enzymes make that chemistry possible. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 10 of 63 How we study mechanisms There are a variety of experimental tools available for understanding mechanisms, including isotopic labeling of substrates, structural methods, and spectroscopic kinetic techniques. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 11 of 63 Ionic reactions Define them as reactions that involve charged, or at least polar, intermediates Typically 2 reactants Electron rich (nucleophilic) reactant Electron poor (electrophilic) reactant Conventional to describe reaction as attack of nucleophile on electrophile Drawn with nucleophile donating electron(s) to electrophile 10/23/2008 Biochemistry: Enzyme Mechanisms p. 12 of 63 Attack on Acyl Group Transfer of an acyl group: scheme 6.1 Nucleophile Y attacks carbonyl carbon, forming tetrahedral intermediate X- is leaving group 10/23/2008 Biochemistry: Enzyme Mechanisms p. 13 of 63 Direct Displacement Attacking group adds to face of atom opposite to leaving group (scheme 6.2) Transition state has five ligands; inherently less stable than scheme 6.1 10/23/2008 Biochemistry: Enzyme Mechanisms p. 14 of 63 Cleavage Reactions Both electrons stay with one atom Covalent bond produces carbanion: R3—C—H R3—C:- + H+ Covalent bond produces carbocation: R3—C—H R3—C+ + :H- One electron stays with each product Both end up as radicals R1O—OR2 R1O• + •OR2 Radicals are highly reactive— some more than others 10/23/2008 Biochemistry: Enzyme Mechanisms p. 15 of 63 Oxidation-Reduction Reactions Commonplace in biochemistry: EC 1 Oxidation is a loss of electrons Reduction is the gain of electrons In practice, often: oxidation is decrease in # of C-H bonds; reduction is increase in # of C-H bonds Intermediate electron acceptors and donors are organic moieties or metals Ultimate electron acceptor in aerobic organisms is usually dioxygen (O2) 10/23/2008 Biochemistry: Enzyme Mechanisms p. 16 of 63 Biological redox reactions Generally 2-electron transformations Often involve alcohols, aldehydes, ketones, carboxylic acids, C=C bonds: R1R2CH-OH + X R1R2C=O + XH2 R1HC=O + X + OH- R1COO- + XH2 X is usually NAD, NADP, FAD, FMN A few biological redox systems involve metal ions or Fe-S complexes Usually reduced compounds are higher-energy than the corresponding oxidized compounds 10/23/2008 Biochemistry: Enzyme Mechanisms p. 17 of 63 Overcoming the barrier Free Energy Simple system: single high-energy transition state intermediate between reactants, products G‡ R P Reaction Coordinate 10/23/2008 Biochemistry: Enzyme Mechanisms p. 18 of 63 Intermediates Often there is a quasi-stable intermediate state midway between reactants & products; transition states on either side Free Energy T2 T1 I R P Reaction Coordinate 10/23/2008 Biochemistry: Enzyme Mechanisms p. 19 of 63 Activation energy & temperature It’s intuitively sensible that higher temperatures would make it easier to overcome an activation barrier Rate k(T) = Q0exp(-G‡/RT) G‡ = activation energy or Arrhenius energy This provides tool for measuring G‡ 10/23/2008 Biochemistry: Enzyme Mechanisms Svante Arrhenius p. 20 of 63 Determining G‡ ln k Remember k(T) = Q0exp(-G‡/RT) ln k = lnQ0 - G‡/RT Measure reaction rate as function of temperature Plot ln k vs 1/T; slope will be -G‡/R 1/T, K-1 10/23/2008 Biochemistry: Enzyme Mechanisms p. 21 of 63 How enzymes alter G‡ Enzymes reduce G‡ by allowing the binding of the transition state into the active site Binding of the transition state needs to be tighter than the binding of either the reactants or the products. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 22 of 63 G‡ and Entropy Effect is partly entropic: When a substrate binds, it loses a lot of entropy. Thus the entropic disadvantage of (say) a bimolecular reaction is soaked up in the process of binding the first of the two substrates into the enzyme's active site. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 23 of 63 Enthalpy and transition states Often an enthalpic component to the reduction in G‡ as well Ionic or hydrophobic interactions between the enzyme's active site residues and the components of the transition state make that transition state more stable. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 24 of 63 Two ways to change G‡ Reactants bound by enzyme are properly positioned Get into transitionstate geometry more readily E A B A+B Transition state is stabilized E A B A+B A-B 10/23/2008 Biochemistry: Enzyme Mechanisms A-B p. 25 of 63 Reactive sidechains in a.a.’s AA Group Charge @pH=7 Asp —COO-1 Glu —COO-1 His Imidazole ~0 Cys —CH2SH ~0 Tyr Phenol 0 Lys NH3+ +1 Arg guanadinium +1 Ser —CH2OH 0 10/23/2008 Functions Cation binding, H+ transfer Same as above Proton transfer Covalent binding of acyl gps H-bonding to ligands Anion binding, H+ transfer Anion binding See cys Biochemistry: Enzyme Mechanisms p. 26 of 63 Generalizations about activesite amino acids Typical enzyme has 2-6 key catalytic residues His, asp, arg, glu, lys account for 64% Remember: pKa values in proteins sometimes different from those of isolated aa’s Frequency overall Frequency in catalysis 10/23/2008 Biochemistry: Enzyme Mechanisms p. 27 of 63 Cleavages by base Simple cleavage: —X—H + :B —X:- + H—B+ This works if X=N,O; sometimes C Removal of proton from H2O to cleave C-X: O O O —C—N —C—OH + HN —C—N HO O H H :B 10/23/2008 H—B+ Biochemistry: Enzyme Mechanisms :B p. 28 of 63 Cleavage by acid Covalent bond may break more easily if one of its atoms is protonated Formation of unstable intermediate, R-OH2+, accelerates the reaction Example: R+ + OH- R—OH R—OH2+ (Slow) (Fast) R+ + H2O 10/23/2008 Biochemistry: Enzyme Mechanisms p. 29 of 63 Covalent catalysis Reactive side-chain can be a nucleophile or an electrophile, but nucleophile is more common A—X + E X—E + A X—E + B B—X + E Example: sucrose phosphorylase Net reaction: Sucrose + Pi Glucose 1-P + fructose Fructose=A, Glucose=X, Phosphate=B 10/23/2008 Biochemistry: Enzyme Mechanisms p. 30 of 63 Rates often depend on pH If an amino acid that is necessary to the mechanism changes protonation state at a particular pH, then the reaction may be allowed or disallowed depending on pH Two ionizable residues means there may be a narrow pH optimum for catalysis 10/23/2008 Biochemistry: Enzyme Mechanisms p. 31 of 63 Papain as an example 1 relative reaction rate Papain pH-rate profile Cys-25 His-159 0 2 3 4 5 6 7 8 9 10 11 pH 10/23/2008 Biochemistry: Enzyme Mechanisms p. 32 of 63 Diffusion-controlled reactions Some enzymes are so efficient that the limiting factor in completion of the reaction is diffusion of the substrates into the active site: These are diffusion-controlled reactions. Ultra-high turnover rates: kcat ~ 109 s-1. We can describe kcat / Km as catalytic efficiency of an enzyme. A diffusioncontrolled reaction will have a catalytic efficiency on the order of 108 M-1s-1. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 33 of 63 Triosephosphate isomerase (TIM) dihydroxyacetone phosphate glyceraldehyde-3-phosphate Glyc-3-P DHAP 10/23/2008 Km=10µM kcat=4000s-1 kcat/Km=4*108M-1s-1 Biochemistry: Enzyme Mechanisms p. 34 of 63 TIM mechanism DHAP carbonyl H-bonds to neutral imidazole of his-95; proton moves from C1 to carboxylate of glu165 Enediolate intermediate (C—O- on C2) Imidazolate (negative!) form of his95 interacts with C1—O-H) glu165 donates proton back to C2 See Fort’s treatment or fig. 6.7. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 35 of 63 Examining enzyme mechanisms will help us understand catalysis Examining general principles of catalytic activity and looking at specific cases will facilitate our appreciation of all enzymes 10/23/2008 Biochemistry: Enzyme Mechanisms p. 36 of 63 Binding modes: proximity We describe enzymatic mechanisms in terms of the binding modes of the substrates (or, more properly, the transition-state species) to the enzyme. One of these involves the proximity effect, in which two (or more) substrates are directed down potential-energy gradients to positions where they are close to one another. Thus the enzyme is able to defeat the entropic difficulty of bringing substrates together. 10/23/2008 Biochemistry: Enzyme Mechanisms William Jencks p. 37 of 63 Binding modes: efficient transition-state binding Transition state fits even better (geometrically and electrostatically) in the active site than the substrate would. This improved fit lowers the energy of the transition-state system relative to the substrate. Best competitive inhibitors of an enzyme are those that resemble the transition state rather than the substrate or product. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 38 of 63 Adenosine deaminase with transition-state analog Transition-state analog: Ki~10-8 * substrate Km Wilson et al (1991) Science 252: 1278 QuickTime™ and a TIFF (LZW) decompressor are needed to see this picture. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 39 of 63 Induced fit Refinement on original Emil Fischer lock-and-key notion: both the substrate (or transitionstate) and the enzyme have flexibility Binding induces conformational changes 10/23/2008 Biochemistry: Enzyme Mechanisms p. 40 of 63 Example: hexokinase Glucose + ATP Glucose-6-P + ADP Risk: unproductive reaction with water Enzyme exists in open & closed forms Glucose induces conversion to closed form; water can’t do that Energy expended moving to closed form 10/23/2008 Biochemistry: Enzyme Mechanisms p. 41 of 63 Hexokinase structure Diagram courtesy E. Marcotte, UT Austin 10/23/2008 Biochemistry: Enzyme Mechanisms p. 42 of 63 Tight binding of ionic intermediates Quasi-stable ionic species strongly bound by ion-pair and H-bond interactions Similar to notion that transition states are the most tightly bound species, but these are more stable 10/23/2008 Biochemistry: Enzyme Mechanisms p. 43 of 63 Serine protease mechanism Only detailed mechanism that we’ll ask you to memorize One of the first to be elucidated Well studied structurally Illustrates many other mechanisms Instance of convergent and divergent evolution 10/23/2008 Biochemistry: Enzyme Mechanisms p. 44 of 63 The reaction Hydrolytic cleavage of peptide bond Enzyme usually works on esters too Found in eukaryotic digestive enzymes and in bacterial systems Widely-varying substrate specificities Some proteases are highly specific for particular aas at position 1, 2, -1, . . . Others are more promiscuous CH NH 10/23/2008 R1 NH C C CH NH O R-1Mechanisms Biochemistry: Enzyme p. 45 of 63 Mechanism Active-site serine —OH … Without neighboring amino acids, it’s fairly non-reactive becomes powerful nucleophile because OH proton lies near unprotonated N of His This N can abstract the hydrogen at nearneutral pH Resulting + charge on His is stabilized by its proximity to a nearby carboxylate group on an aspartate side-chain. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 46 of 63 Catalytic triad The catalytic triad of asp, his, and ser is found in an approximately linear arrangement in all the serine proteases, all the way from non-specific, secreted bacterial proteases to highly regulated and highly specific mammalian proteases. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 47 of 63 Diagram of first three steps 10/23/2008 Biochemistry: Enzyme Mechanisms p. 48 of 63 Diagram of last four steps Diagrams courtesy University of Virginia 10/23/2008 Biochemistry: Enzyme Mechanisms p. 49 of 63 Chymotrypsin as example Catalytic Ser is Ser195 Asp is 102, His is 57 Note symmetry of mechanism: steps read similarly L R and R L Diagram courtesy of Anthony Serianni, University of Notre Dame 10/23/2008 Biochemistry: Enzyme Mechanisms p. 50 of 63 Oxyanion hole When his-57 accepts proton from Ser-195: it creates an R—O- ion on Ser sidechain In reality the Ser O immediately becomes covalently bonded to substrate carbonyl carbon, moving - charge to the carbonyl O. Oxyanion is on the substrate's oxygen Oxyanion stabilized by additional interaction in addition to the protonated his 57: main-chain NH group from gly 193 H-bonds to oxygen atom (or ion) from the substrate, further stabilizing the ion. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 51 of 63 Oxyanion hole cartoon Cartoon courtesy Henry Jakubowski, College of St.Benedict / St.John’s University 10/23/2008 Biochemistry: Enzyme Mechanisms p. 52 of 63 Modes of catalysis in serine proteases Proximity effect: gathering of reactants in steps 1 and 4 Acid-base catalysis at histidine in steps 2 and 4 Covalent catalysis on serine hydroxymethyl group in steps 2-5 So both chemical (acid-base & covalent) and binding modes (proximity & transition-state) are used in this mechanism 10/23/2008 Biochemistry: Enzyme Mechanisms p. 53 of 63 Specificity Active site catalytic triad is nearly invariant for eukaryotic serine proteases Remainder of cavity where reaction occurs varies significantly from protease to protease. In chymotrypsin hydrophobic pocket just upstream of the position where scissile bond sits This accommodates large hydrophobic side chain like that of phe, and doesn’t comfortably accommodate hydrophilic or small side chain. Thus specificity is conferred by the shape and electrostatic character of the site. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 54 of 63 Chymotrypsin active site Comfortably accommodates aromatics at S1 site Differs from other mammalian serine proteases in specificity Diagram courtesy School of Crystallography, Birkbeck College 10/23/2008 Biochemistry: Enzyme Mechanisms p. 55 of 63 Divergent evolution Ancestral eukaryotic serine proteases presumably have differentiated into forms with different side-chain specificities Chymotrypsin is substantially conserved within eukaryotes, but is distinctly different from elastase 10/23/2008 Biochemistry: Enzyme Mechanisms p. 56 of 63 iClicker quiz! Why would the nonproductive hexokinase reaction H2O + ATP -> ADP + Pi be considered nonproductive? (a) Because it needlessly soaks up water (b) Because the enzyme undergoes a wasteful conformational change (c) Because the energy in the high-energy phosphate bond is unavailable for other purposes (d) Because ADP is poisonous (e) None of the above 10/23/2008 Biochemistry: Enzyme Mechanisms p. 57 of 63 iClicker quiz, question 2: Why are proteases often synthesized as zymogens? (a) Because the transcriptional machinery cannot function otherwise (b) To prevent the enzyme from cleaving peptide bonds outside of its intended realm (c) To exert control over the proteolytic reaction (d) None of the above 10/23/2008 Biochemistry: Enzyme Mechanisms p. 58 of 63 Question 3: what would bind tightest in the TIM active site? (a) DHAP (substrate) (b) D-glyceraldehyde (product) (c) 2-phosphoglycolate (Transition-state analog) (d) They would all bind equally well 10/23/2008 Biochemistry: Enzyme Mechanisms p. 59 of 63 Convergent evolution Reappearance of ser-his-asp triad in unrelated settings Subtilisin: externals very different from mammalian serine proteases; triad same 10/23/2008 Biochemistry: Enzyme Mechanisms p. 60 of 63 Subtilisin mutagenesis Substitutions for any of the amino acids in the catalytic triad has disastrous effects on the catalytic activity, as measured by kcat. Km affected only slightly, since the structure of the binding pocket is not altered very much by conservative mutations. An interesting (and somewhat non-intuitive) result is that even these "broken" enzymes still catalyze the hydrolysis of some test substrates at much higher rates than buffer alone would provide. I would encourage you to think about why that might be true. 10/23/2008 Biochemistry: Enzyme Mechanisms p. 61 of 63 Cysteinyl proteases Ancestrally related to ser proteases? Cathepsins, caspases, papain Contrasts: Cys —SH is more basic than ser —OH Residue is less hydrophilic S- is a weaker nucleophile than O- 10/23/2008 Diagram courtesy of Mariusz Jaskolski, U. Poznan Biochemistry: Enzyme Mechanisms p. 62 of 63 Papain active site Diagram courtesy Martin Harrison, Manchester University 10/23/2008 Biochemistry: Enzyme Mechanisms p. 63 of 63