* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lesch-Nyhan Syndrome
Two-hybrid screening wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Catalytic triad wikipedia , lookup
Genetic code wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Biosynthesis wikipedia , lookup
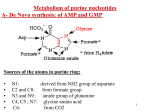
Lesch-Nyhan Syndrome • • • • Nucleic acid turnover (synthesis and degradation) is an ongoing metabolic process in most cells. Messenger RNA in particular is actively synthesized and degraded. These degradative processes can lead to the release of free purines in the form of adenine, guanine, and hypoxanthine (the base in IMP). These substances represent a metabolic investment by cells. Socalled salvage pathways exist to recover them in useful form. Salvage reactions involve resynthesis of nucleotides from bases via phosphoribosyltransferases. Base + PRPP -----nucleoside-5'-phosphate + PPi The subsequent hydrolysis of PPi to inorganic phosphate by pyrophosphatases renders the phosphoribosyltransferase reaction effectively irreversible. The purine phosphoribosyltransferases are adenine phosphoribosyltransferase (APRT), which mediates AMP formation, and hypoxanthine-guanine phosphoribosyltransferase (HGPRT), which can act on either hypoxanthine to form IMP or guanine to form GMP • • Lesch-Nyhan Syndrome: HGPRT Deficiency Leads to Severe Clinical Disorder The symptoms of Lesch-Nyhan syndrome are tragic: a crippling gouty arthritis due to excessive uric acid accumulation and, worse, severe malfunctions in the nervous system that lead to mental retardation, spasticity, aggressive behavior, and selfmutilation. Lesch-Nyhan syndrome results from a complete deficiency in HGPRT activity. The structural gene for HGPRT is located on the X chromosome, and the disease is a congenital, recessive, sex-linked trait manifested only in males. The severe consequences of HGPRT deficiency argue that purine salvage has greater metabolic importance than simply the energy-saving recovery of bases. Although HGPRT might seem to play a minor role in purine metabolism, its absence has profound consequences: de novo purine biosynthesis is dramatically increased and uric acid levels in the blood are elevated. Presumably, these changes ensue because lack of consumption of PRPP by HGPRT elevates its availability for glutamine-PRPP amidotransferase, enhancing overall de novo purine synthesis and, ultimately, uric acid production (Figure 27.8). Despite these explanations, it remains unclear why deficiency in this single enzyme leads to the particular neurological aberrations characteristic of the syndrome. Fortunately, deficiencies in HGPRT activity in fetal cells can be detected following amniocentesis. • Crystal structures have been determined for free Escherichia coli hypoxanthine phosphoribosyltransferase (HPRT) (2.9 Å resolution) and for the enzyme in complex with the reaction products, inosine 5`monophosphate (IMP) and guanosine 5`monophosphate (GMP) (2.8 Å resolution). (A) Two orthogonal views of the structure of subunit A from the E. coli HPRT-GMP complex. The βstrands, shown as direction arrows are yellow in the core domain and pink in the hood domain. The mobile loop, which includes residues 73–82, is not observed in the crystal structure. To complete the structure, a hypothetical mobile loop has been modeled in and depicted as white coil. The GMP molecule is drawn as solid spheres and the atoms colored green for carbon, blue for nitrogen, red for oxygen, and pink for phosphorous. (B) The structure of the E. coli HPRT-GMP tetramer viewed down the crystallographic twofold axes. (C) The active site of subunit A of E. coli HPRT. (Top) The IMP complex. (Middle) The GMP complex. (Bottom) Free enzyme showing bound water molecules (red) and Mg2+ (pink) as solid spheres. • Data reported to GenBankTM indicate that the HPRTs of distantly related organisms share extensive primary sequence homology. For example, there is 41% identity for amino acids in the human and a bacterial HPRT. However, among well over 20 HPRT sequences reported there are only 9 invariant amino acids and all but the HPRT of Giardia lamblia also are invariant at Glu-133 and Asp-134 (Table ). In general, conserved residues of HPRTs and bacterial XPRTs differ at positions homologous with human Leu-67 and Glu-133 (Ser-36 and Asp-88 in the XPRT of Escherichia coli). Solutions for the crystal structures of HPRTs reveal that the 11 conserved residues immediately flank or are very near the active site of HPRTs. • If the crystal structures of all purine PRTs are analyzed together with the amino acid sequences reported to GenBankTM, there are only 2 residues (corresponding with human Gly69 and Asp-134) that are clearly invariant. A Gly69Glu mutation virtually inactivates the human HPRT, resulting in Lesch-Nyhan syndrome, whereas a D134G mutation partially inactivates the enzyme, resulting in gouty arthritis . The invariant glycine may be essential for the formation of a tight turn and an unusual non-proline cis-peptide in active site. • An aspartate at position 193 of HPRTs has been shown to participate indirectly in binding pyrophosphate and purines via the formation of a direct protein metal bond with a magnesium ion designated M2. Asp-193 also forms hydrogen bonds with two water molecules coordinated by the metal, and the metal forms coordinated interactions with two oxygens of PRPP or PPi. A third coordinated water molecule forms another hydrogen bond with the N-3 atom of purine substrates. • An invariant arginine at position 199 of HPRTs participates directly in binding pyrophosphate and may contribute to positioning both substrates by being close enough to the carboxyl group of Asp-193 to affect its position. Together, these interactions help to position both substrates for in-line nucleophilic attack at the C1′ carbon of PRPP or a nucleotide. A Asp193Asn mutation virtually inactivates the human enzyme resulting in Lesch-Nyhan's syndrome . La sindrome è determinata da diversi tipi di mutazione che ne determinano anche la gravità • Different HPRT1 mutations result in varied levels of residual HGprt enzyme activity as well as a spectrum of disease characteristics. Classic features of LND include hyperuricemia and its sequelae (gout, nephrolithiasis,), motor disability (dystonia, chorea, and spasticity), intellectual impairment, and self-injurious behavior • We identiWed ten patients from eight diVerent families all with the c.143G>A mutation. All exhibited signs of uric acid overproduction. Nine were classiWed as HND because of evidence for motor or cognitive impairment, while one was classiWed as HRH. The age at presentation varied from infancy to 28 years. • The biochemical properties of the 143G>A mutation leading to arg48his were compared with normal human • • • • • • • • • • • • • • Although early studies suggested that mutations encoding the active site of the enzyme would be associated most closely with disease, subsequent studies demonstrated that mutations were spread throughout the gene and upstream regulatory sequences (Jinnah et al. 2000, Jinnah et al. 2004). Presumably, mutations distant from the active site aVect enzyme activity by interfering with protein expression, stability, dimerization or by causing other deleterious conformational changes (Duan et al. 2004). While there is no obvious correlation between the location of gene mutations and the clinical phenotype, the predicted consequence of the mutation does correlate with clinical phenotype (de Gemmis et al. 2010; Jinnah et al. 2000, 2004; Jurecka et al. 2008)