* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download dhaA - Queen`s University Belfast
Electron transport chain wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Transformation (genetics) wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biosynthesis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Metalloprotein wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
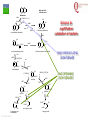
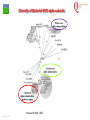
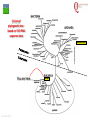
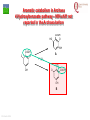
From Biosphere to Molecule via The Petri Dish M. J. Larkin The QUESTOR Centre and Biological Sciences The Queen’s University of Belfast ©M J Larkin 2008. Summary • • • The lab and acknowledgements Overview - the planet and its microbial biomass The methodological approach - four examples of research done – pure linked to applied..... • • • • ©M J Larkin 2008. Chloroalkane degradation - chlorobutane and methyl chloride Oxygenases in biodegradation Archaea and oxidative catabolism – extreme environments Bioremediation and microbial diversity ACKNOWLEDGEMENTS - Microbiology Laboratory QUESTOR Centre – Collaborators - current and recent inmates Leoinid Kulakov and Chris Allen John Quinn Sheila Patrick (Medicine and Dentistry) Johannes Barth, Jim Hall (Bob Kalin, Trevor Elliot, Civ’ Eng’) Cathy Coulter (David Harper, Jack Hamilton , Agriculture) Dave Clarke, Gwen O’Reilly (Derek Boyd, Chemistry) Joe Vyle (Chemistry); Peter Coyle (RVL); Stephen Allen (Chem Eng) Andrew Ferguson Derek Fairley Dave Lipscomb Helen Irvine Ros Andserson Andrew Lee Nichola Connery Andrew Mudd Harpinder Mundi Antonio de Casale Jose Argudo ©M J Larkin 2008. Ian Thompson, Andrew Whitely, Wei Huang, Oxford Dick Janssen, Gerrit Poelarends GRONINGEN Andy Weightman, Julian Marchesi CARDIFF Andrei Filonov, Vladimir Ksenzenko PUSHCHINO David Gibson, Ramaswamy, Rebecca Parales: U of IOWA Ian Pepper and Chris Rensing, John O’Hanlon: Water Quality Center, U of ARIZONA VISITORS: Samera Alwadi; KUWAIT Susheela Carroll; U of Arizona Sebastian Sorensen; GEUS Denmark Monika Knoppova; ICT Prague Asa Moyce Emma Frew Peter Gray Andrew Fraser Kathryn Lawson Veronique Durocq Chen Shenchang Tim Gilfedder Paul Flanagan Osa Osalador FUNDING SOURCES: INDUSTRY:QUESTOR Centre: Exxon: ICI:DuPont: ESB; Shell: BP SRIF: ECFW4:EC TDP: PEACE II Centres of Excellence: INTAS: BBSRC: DTI: LINK: EPSRC; NERC: DEL CAST: Prospect Globe Award; TALENT; Kuwait Government The Queen’s University Environmental Science and TechnolOgy Research Centre Jim Swindall Wilson McGarel ©M J Larkin 2008. SCOPE OF THE INTERDISCIPLINARY RESEARCH EFFORT FROM THE FIELD AND LABORATORY MICROCOSM TO MOLECULAR MICROBIOLOGY; ENZYMES, CELLS AND GENE EVOLUTION AND DIVERSITY BH7-7 BH2 abcd abcd M A B Figure 3: 2D-PAGE gels of regions selected from the ‘other fraction’ – not containing the NarAa and Ab components – but containing putative reductase and ferredoxin components - from a MonoQ column. A– pyruvate –grown cells without induction of naphthalene associated genes; B- naphthalene- grown cells with induction of naphthalene associated genes. Examples of proteins associated with naphthalene degradation are indicated with arrows. ©M J Larkin 2008. RESEARCH AREAS • • • • • • • • ©M J Larkin 2008. Molecular Biology/Genetics - Biochemistry of Biodegradation - and Biotransformations. Mobile genetic elements – insertion sequences Soil bacteria – Rhodococcus - Genetic systems and regulation Extremophiles (Salinity/pH) Naphthalene dioxygenase - evolution and mechanism haloalkane dehalogenases Waste water treatment - Sludge bulking and Microthrix Contaminated land remediation – isotope probing Microorganisms - the root of diversity. Eubacteria 3.5 billion years ©M J Larkin 2008. Plants & Animals Archaea Where are they found? Biomass on the planet. • • • • ©M J Larkin 2008. Most culturing analysis misses over 99% of the microbial population Molecular techniques now reveal hidden diversity Heterotrophs 5-20% biomass in sea waters Rich bacterial communities in sub-surface strata (600 m deep) • up to 2 x 1040 tons - more than all flora and fauna • equivalent up to 2 m layer over planet! The potential of One gram of soil… • • • • • 1 x 1010 microbial cells (typical clay loam) 4 x 103 microbial ‘species’ < 0.1% can be cultivated in vitro (so far…) Many groups known only from DNA sequence data Only 1 or 2 cultivated members of some diverse taxonomic orders are known ©M J Larkin 2008. Philosophy of the laboratory mission • Traditional approach: Millions of chemicals – 10 x 106 Chemicals • • • • 8 x 106 Xenobiotic 1 x 106 Recalcitrant 0.4 x 106 traded at over 50 tonnes per year Toxicological/ biodegradative data on only around 5000-6000 – Pick one - get a degrader - define catabolism - look in situ. – Cultivate – Research and Publish • Alternative approach – Look at environment and population diversity - set out to isolate specific dominant groups - define novel catabolism - look for activity in situ – Rhodococcus – Haloarchaea - Alkaliphiles ©M J Larkin 2008. Title-page of:, Instauratio Magna (1620) Francis Bacon which contained his Novum Organon “On the state of Sciences that is neither prosperous nor far advanced… Men (sic) seem to have no good sense of either their resources or their power: but to exaggerate the former and underrate the latter. "Multi pertransibunt et augebitur scientia“ (Many will pass through and knowledge will be increased). Book of Daniel (chapter 12, verse 4) ©M J Larkin 2008. Hence either they put an insane value on the Arts which they already have and look no further or, undervaluing themselves, they waste their power on trifles and fail to try out things which go to the heart of the matter. And so they are like the fatal pillars of Hercules to the Sciences; for they are not stirred by the desire or hope of going further.” Aerobic biodegradability of some common pollutants From: Dick B. Janssen, Inez J. T. Dinkla, Gerrit J. Poelarends and Peter Terpstra Bacterial degradation of xenobiotic compounds: evolution and distribution of novel enzyme activities. Environmental Microbiology (2005) 7: 1868–1882 ©M J Larkin 2008. Fate of chloroalkanes: 1- Chlorobutane and Chloromethane • • • • • • • ©M J Larkin 2008. Chloalkanes very commonly used in industry in a wide range of processes. 1-chlorobutane a good model substrate to investigate the biodegradation mechanisms possible. Chloromethane (CH3Cl): most abundant volatile halocarbon in the atmosphere. Amospheric concentration: 600 parts per 1012 : 5 million metric tons. Ozone destruction - 15 to 20% - natural origin – not industrial: e.g. wood-rot fungi Biodegradative fate only more recently investigated Same mechanism as other haloalkanes? 1- Chlorobutane degradation by Rhodococcus sp NCIMB13064 CH2-Cl H O HCl CH2-OH 2 X XH2 CH2 CH2 CH2 CH2 CH3 DhaA CH3 AdhA CHO COOH Y +H2O YH2 CH2 CH2 CH2 CH2 CH3 CH3 AldA Order of genes on pRTL1 (approx 100 Kbp plasmid) IS2112 invA dhaR dhaA adhA 1 Kb ©M J Larkin 2008. aldA Global Dha A spread in bacterial isolates Gerrit J. Poelarends, Marjan Zandstra, Tjibbe Bosma, Leonid A. Kulakov, Michael J. Larkin, Julian R. Marchesi, Andrew J. Weightman, and Dick B. Janssen (2000)Haloalkane-Utilizing Rhodococcus Strains Isolated from Geographically Distinct Locations Possess a Highly Conserved Gene Cluster Encoding Haloalkane Catabolism. J.Bacteriol. 182:2725-2731. ©M J Larkin 2008. Spread of dhaA amongst strains world-wide NCIMB13064 TB2 m15-3 UK USA JAPAN IS2112 invA GJ70 HA1 Y2 dhaR dhaA adhA aldA THE NETHERLANDS SWITZERLAND UK invA dhaR dhaA adhA aldA dhaA (100%) also in Pseudomonas pavonaceae 170 The Netherlands (1,3-dichloropropene) Poelarends Janssen et al 1999 Appl.Environ. Microbiol. 64:2931-2936 ©M J Larkin 2008. dhaA : Recombinations across species Mycobacterium sp GP1 (1,2-dibromoethane) intM invA dhaR dhaAf Rhodococcus sp NCIMB 13064 IS2112 P .pavonaceae 170 (1,3-dichlrororopene) ©M J Larkin 2008. invA intP dhaR dhaA adhA aldA dhaA tnpA IS1071 Genetic Recombinations and Global Distribution of Dehalogenases - Summary Mycobacterium sp GP1 (1,2-dibromoethane) intM P .pavonaceae 170 (1,3-dichlrororopene) Rhodococcus sp NCIMB 13064: UK TB2: USA m15-3: JAPAN Rhodococcus sp GJ70:THE NETHERLANDS HA1: SWITZERLAND Y2: UK ©M J Larkin 2008. invA dhaR intP IS2112 invA invA dhaAf dhaA dhaR dhaR dhaA dhaA tnpA adhA adhA IS1071 aldA aldA Isolation of chloromethane degrader CC495 Aminobacter lissarensis CATHERINE COULTER, JOHN T. G. HAMILTON, W. COLIN MCROBERTS, LEONID KULAKOV,MICHAEL J. LARKIN,AND DAVID B. HARPER (1999) Halomethane:Bisulfide/Halide Ion Methyltransferase, an Unusual Corrinoid Enzyme of Environmental Significance Isolated from an Aerobic Methylotroph Using Chloromethane as the Sole Carbon Source. APPLIED AND ENVIRONMENTAL MICROBIOLOGY, 65: 4301–4312. ©M J Larkin 2008. Methyl transferase activity – not halohydrolase Methanethiol HS- ©M J Larkin 2008. Role of Oxygen in the biosphere • • • • • • For many compounds to be degraded quickly there needs to be a reaction with Oxygen. Known as Oxygen fixation Mediated in nature my many microorganisms Enzymes known as oxygenases Carbon and Oxygen cycle at necessary for life on the planet Fortunately molecular Oxygen is not very reactive ©M J Larkin 2008. The reactivity of Oxygen • • • Oxygen in the air is in its "ground“ state - 3O2. Outermost pair of electrons have parallel spins (↑↑ ) - "triplet" state. This does not allow them to react with most molecules – just as well !!! – SPIN FORBIDDEN. • • ©M J Larkin 2008. However, triplet oxygen can be activated by the addition of energy, and transformed into reactive oxygen species. Outermost pair of electrons have antiparallel spins (↓↑ ) - "singlet" state. Activation of Oxygen enzymatically Not common in catabolism Very common in oxygenases ©M J Larkin 2008. Microbial Oxygenases and Oxygen For most compounds to be degraded they must react with O2 Mediated by bacteria in the environment at low temperature using iron in diverse enzymes • • • • • • • ©M J Larkin 2008. This is facilitated by oxygenases Two types Mono- add one -OH group Di- add TWO -OH groups The “corner-stone” of the C and O cycle in nature. Naphthalene dioxygenase NDO - well studied in Pseudomonas The current paradigm NarA and NarB in Rhodococcus Naphthalene NahA H NADH + O2 + H+ OH NAD+ OH H OH NAD+ Scheme for naphthalene catabolism in bacteria OH NahB NADH + H+ 1,2-dihydroxynaphthalene cis-naphthalene dihydrodiol O2 NahC OH O COOH NahD COOH OH O cis-o-hydroxybenzalpyruvate 2-hydroxychromene-2-carboxylate H2O NahE CH3COCOOOH NAD+ NADH + H+ CHO NahF salicylaldehyde OH H+, NADH,O2 NADH,O2,ATP,CoA COOH salicylate NAD+ S1H RING OPENINING DIOXYGENASES NAD+ S5H OH HO OH COOH OH catechol C23O CHO COOH OH 2-hydroxymuconic semialdehyde ©M J Larkin 2008. RING HYDROXYLATING DIOXYGENASE O2 O2 gentisate C12O O2 HOOC G12O HOOC O OH HOOC cis,cis-muconic acid HOOC maleylpyruvate What are the potential rate-limiting steps? Bioavailability Solubility Substrate fit Natural vs pollutant Affinity and rate Cellular metabolism DIOXYGENASE BOTTLENECK Provision of oxygen EnvironmentAffinity and rate ©M J Larkin 2008. Provision of electrons Rhodococcus NDO characterisation • • • • • • • ©M J Larkin 2008. NCIMB 12038 Enzyme components purified Novel Naphthalene dioxygenase (NDO) N-terminal sequences DNA and amino acid sequences Key active site aa’s conserved Present in other strains Comparison of and components of ISPNAR (Rhodococcus NDO) and ISPNAH (Pseudomonas NDO) *Analogous Rhodococcus ISPNAR Napthalene Pyruvate Salicylate 55KD 23KD NAD+ **Pseudomonas ISPNAH Reductase NAP Ferredoxin (OX) NAP (OX) ISP NAP (OX) O2 NADH + H+ Reductase FerredoxinNAP NAP (RED) (RED) ISP NAP (RED) NO significant DNA HOMOLOGY: Amino acid similarity (31%) (39%) ©M J Larkin 2008. OH OH Conservation of the key amino acids in subunits of NDOs from Rhodococcus and Pseudomonas. Rieske Centre Active site Putative Ferredoxin Electron transfer Region of unknown binding region (Rieske-Act. Site) function NahAc NarAa NahAc NarAa NahAc NarAa NahAc NarAa NahAc NarAa C81 C88 N201 N209 K97 S104 W106 W113 T299 T297 C101 C108 H208 H216 G98 H105 V117 V124 V300 V298 H83 H90 H213 H221 V100 R107 R84 R91 F301 F299 H104 H111 D205* D213 Q115 V122 E200 D208 P302 P300 D362 D372 S116 G123 N303 N301 P118** P125** W211 M219 *Asp205 is probably important for electron transfer (12) and is essential for activity (18); **Pro118 (as well as Trp211) is from the catalytic domain. ©M J Larkin 2008. Diversity of Bacterial NDO alpha subunits Moser and Stahl, 1999 ©M J Larkin 2008. STRUCTURE OF Rhodococcus NDO ©M J Larkin 2008. Organisation of naphthalene degradation genes in Rhodococcus I24 nidA B C narAa Ab B Ab B D P400 rub1 narR1 R2 narK C oxiA P200 rub1 narR1,R2 rub1 narR1,R2 narK narAa narK narAa NCBI12038 rub2 Ab C B ΔC orf1 – 3 orf4 – 6 CIR2 orf1 rnoA1 A2 A3 A4 rnoB Transcription induced by growth on Naphthalene: narR1, R2 ©M J Larkin 2008. rub2 narK narAa, Ab, B narC orf1 – 3 orf4 – 6 A novel mechanism for electron transfer.... B H OH OH H OH OH OH OH H H NarB eNarAa O2 ©M J Larkin 2008. OH OH H H OH OH NarB NADH + H+ NAD+ NarK NarAa NarAb O2 NarK NarAb Extremophiles – BIODEGRADATION UNDER EXTREME CONDITIONS • Many industrial waste and environmental have: • Extremes of pH – often very caustic waste • Extremes of salinity • Alkaliphile capabilities – • Exxon – Mobil – caustic waste • Halophile capabilities – biodegradation of aromatic compounds • ICI and Water Quality Centre – University of Arizona ©M J Larkin 2008. ‘Universal’ phylogenetic tree based on 16S rRNA sequence data Halobacteriales You are here… ©M J Larkin 2008. Animals 97 Haloarcula vallismortis 92 97 Novel ‘extremely Haloarcula argentinensis 100 Haloarcula sp. D1 100 halophilic Archaea’ Haloarcula hispanica 100 Haloarcula sp. DSW7 63 Growing on Haloarcula marismortui rrnA 28 Aromatic substrates Haloarcula marismortui rrnB Halorhabdus utahensis Halobacterium salinarum 21 Halococcus morrhuae Nantronococcus occultus 24 100 Nantronobacterium gregoryi 51 Natrialba magadii 59 Natrinema versiforme 89 100 Haloterrigena thermotolerans Nantronomonas pharaonis 100 99 Haloferax volcanii Haloferax sp. D1227 100 100 Haloferax mediterranei Halogeometricum borinquense 75 Halobaculum gomorrense 96 Halorubrum distributum 100 100 91 Halorubrum saccharovorum Halorubrum lacusprofundi Halorubrum sp. E4 Methanospirillum hungatei ©M J Larkin 2008. Aromatic substrates COOH COOH Aerobic growth of Haloarcula sp. D1 benzoic & 4-hydroxybenzoic acids : OH COOH COOH Gentisate 1,2dioxygenase: ©M J Larkin 2008. HO +O2 OH GDO O HOOC OH ??? Accumulation of gentisic acid from 4-hydroxybenzoic acid in Haloarcula sp. D1 cell suspensions O HOOC OH 2.5 2 COOH OH 1.5 COOH OH Concentration (mM) OH 1 0 .5 0 0 10 20 Time (hours) ©M J Larkin 2008. COOH COOH HO 30 OH 4-Hydroxybenzoate pathway Intramolecular carboxyl-group migration / ‘NIH-shift’ ? OH COOH COOH + 1/2O2 OH OH Synthesised 2,6dideutero-4hydroxybenzoic acid : HO COOH D OH D COOH D A + 1/2O2 OH OH D COOH OH B ©M J Larkin 2008. O MeO 1 H 1H-NMR spectra OH 2 6 3 5 HO H 4 H 6 7.4 4 7.2 7.3 3 7.1 ppm 36.406 7.5 Authentic (nondeuterated) standard O MeO 1 H 3 5 HO OH 2 6 4 D H ©M J Larkin 2008. 6.8 3 ppm 0.795 4 6.9 2.071 6 7.0 8.626 7.1 8.680 7.2 Deuterated (methyl)gentisate Aromatic catabolism in Archaea 4-Hydroxybenzoate pathway – NIH-shift not reported in the Archaea before HO COOH D OH D COOH D A + 1/2O2 OH OH D COOH OH B ©M J Larkin 2008. Gasworks Sites: Best source of aromatic catabolic diversity! ©M J Larkin 2008. Nasty toxic environment! ©M J Larkin 2008. Contaminated Ground water! ©M J Larkin 2008. Extent of contamination at SEREBAR remediation site prb1 BGBH11 91700 prb2 prb3 91650 prb21 prb5 91600 prb12 prb8 prb7 prb13 prb10 91550 prb9 prb14 prb23 BGBH10 prb11 91500 prb20 prb16 91450 prb18 prb17 292100 ©M J Larkin 2008. 292150 292200 292250 292300 CLONE PHYLOGENY V1 V2 15F 16F 17F 18F ß-PROTEOBACTERIA -PROTEOBACTERIA; GEOBACTERIACEAE FIRMICUTES; LACTOB ACILLACEAE ß-PROTEOBACTERIA ß-PROTEOBACTERIA; RHODOCYCLUS ß-PROTEOBACTERIA; COMAMONADACEAE ß-PROTEOBACTERIA; NITROSOLOBUS ß-PROTEOBACTERIA; COMAMONADACEAE -PROTEOBACTERIA; GEOBACTERIACEAE ß-PROTEOBACTERIA; RHODOCYCLUS UNKNOWN ß-PROTEOBACTERIA; RHODOCYCLUS ß-PROTEOBACTERIA; RHODOCYCLUS -PROTEOBACTERIA; PSEUDOMONAS ß-PROTEOBACTERIA; RHODOCYCLUS ß-PROTEOBACTERIA; ALCALIGENACEAE ß-PROTEOBACTERIA; COMAMONADACEAE ß-PROTEOBACTERIA ß-PROTEOBACTERIA ß-PROTEOBACTERIA; BURKHOLDERA -PROTEOBACTERIA; PSEUDOMONAS 19F 20F UNKNOWN ß-PROTEOBACTERIA V4 V5 V7 V8 2F 3F 4F 5F 6F 7F 9F 10F 11F 13F 14F ©M J Larkin 2008. PRESUMPTIVE PHYLOGENETIC IDENTIFICATION OF EUBACTERIAL 16s rDNA CLONES FROM DIRECT SOIL DNA SAMPLES ©M J Larkin 2008. CLONE PHYLOGENY 1G 2G 3G 4G 7G 8G 12G 13G 17G 20G 22G 23G 26G 27G 28G 30G 33G 34G 35G 37G 43G 44G 45G 46G 50G 51G 55G 57G 59G 60G 61G 62G 63G -PROTEOBACTERIA; PSEUDOMONAS -PROTEOBACTERIA; METHYLOCOCCACEAE -PROTEOBACTERIA; PSEUDOMONAS UNKNOWN -PROTEOBACTERIA; METHYLOCOCCACEAE UNKNOWN UNKNOWN UNKNOWN UNKNOWN -PROTEOBACTERIA; XANTHOMONAS UNKNOWN UNKNOWN -PROTEOBACTERIA; XANTHOMONAS -PROTEOBACTERIA; PSEUDOMONAS UNKNOWN UNKNOWN -PROTEOBACTERIA; ENTEROBACTERIACEAE -PROTEOBACTERIA; XANTHOMONAS -PROTEOBACTERIA; PSEUDOMONAS UNKNOWN UNKNOWN UNKNOWN -PROTEOBACTERIA; ENTEROBACTERIACEAE UNKNOWN UNKNOWN UNKNOWN UNKNOWN UNKNOWN -PROTEOBACTERIA; PSEUDOMONAS ß-PROTEOBACTERIA; RHODOCYCLUS UNKNOWN -PROTEOBACTERIA; PSEUDOMONAS -PROTEOBACTERIA; METHYLOCOCCACEAE PRESUMPTIVE PHYLOGENETIC IDENTIFICATION OF EUBACTERIAL 16s rDNA CLONES FROM DIRECT GROUNDWATER DNA SAMPLES LABORATORY MICROCOSM REACTIVE BARRIER - removal of key pollutants – aromatic compounds Benzene Profile 11-5-01 Benzene -20.00 Phenol Profile (11-5-01) 0.00 20.00 40.00 60.00 80.00 100.00 120.00 Phenol -20.00 0 0.00 5000 20.00 2,4-Dimethylphenol 10000 15000 20000 Profile 25000 11-5-01 30000 35000 40.00 (ug/L) 60.00 80.00 100.00 120.00 2,4-Dimethylphenol -20.00 0 0.00 4000 20.00 0 ©M J Larkin 2008. 8000 12000 16000 20000 24000 28000 32000 (ug/L) 40.00 60.00 80.00 100.00 60000 120000 180000 240000 300000 360000 (ug/L) 120.00 Microbiological sample points – SEREBAR Onlookers Tool-box When he goes back to his mobile phone, that's when it's Back to the lab again yo This whole rhapsody He better go capture this moment and hope it don't pass him….. Eminem - Lose Yourself ©M J Larkin 2008. Look, if you had one shot, one opportunity To seize everything you ever wanted-One moment Interceptor and Would you capture inlet it or just let it slip? …. Eminem - Lose Yourself Population diversity in the PRB ©M J Larkin 2008. Which organisms are the main degraders ? - Stable Isotope Probing (SIPS) – to detect PAH degraders - naphthalene Using 13C labelled naphthalene Amplification – Sequence analysis = taxonomic and phylogenetic information Functional genes ©M J Larkin 2008. Napthalene Concentration (µM) Utlilisation of 12C and 13C - naphthalene by groundwater bacteria in microcosms 5 4 3 2 1 0 0 10 20 30 40 50 60 70 80 Time (hours) Figure 1. Degradation of 12C- and 13C-naphthalene (3.8 µM) in laboratory microcosm flasks inoculated with groundwater. ©M J Larkin 2008. DGGE and 16s rDNA sequence identification T=0 hr factions 6-15 T=36 hr fraction 6-15 M1 M2 6 7 8 9 10 11 12 13 14 15 6 7 8 9 10 11 12 13 14 15 A GW with 3.8 µM 12C-Naph GW with 3.8 µM 13C-Naph GW only 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 Acidovorax sp – related to Comamonas spp ©M J Larkin 2008. NDO - subunit expression – RT-PCR of 13C-RNA RT-PCR of 13C-RNA fractions Ladder ©M J Larkin 2008. C 6 7 8 9 10 11 12 C Concentration effect on dominant degraders....... GW 3µM 30µM 60µM 300µM 600µM +c -c1 -c2 NDO - subunit Pseudomonas P. putida G7 type NDO - subunit Commonas Ralstonia U2 type Extensive independent study .......... Only Pseudomonas and Rhodococcus strains isolated No Acidovorax or Comamonas related strains cultivated Comamonas – like NDO -subunit genes amplified from groundwater ©M J Larkin 2008. FISH images show microbial degraders in groundwater sample. Red Acidovorax sp Green Pseudomonas sp Purple overall eubacteria ©M J Larkin 2008. 13 C content per cell (Atom%) Raman micro-spectroscopy analysis of single cells 120 100 Acidovorax Pseudomonas 80 20 19 60 40 40 10 20 0 3.8 µm 13 30 µm C Naphthalene concentration Stable isotope based analysis of phylogenetic identity, functional transcripts and metabolic activity in natural microbial populations Wei E. Huang1,2*ψ, Andrew Ferguson3,4, Andrew C. Singer2, Kathryn Lawson4,5, Ian P. Thompson2, Robert M. Kalin3, Michael J. Larkin4,5, Mark J. Bailey1 and Andrew S. Whiteley1* - in press 13C labelled cells have significant red-shift in spectrum (Huang, W. E., Griffiths, R. I., Thompson, I. P., Bailey, M. J., & Whiteley, A. S. (2004) Anal. Chem. 76, 4452-4458_ ©M J Larkin 2008. Acknowledgements Alan Bull – Cardiff Martin Day – Cardiff Werner Arber – Basle Roger Whittenbury – Warwick Heinz Saedler – Cologne Mick Chandler – Toulouse Simon Baumberg – Leeds Howard Dalton – Warwick Gerben Zylstra – Rutgers Chris Knowles – Oxford Julian Davies - Vancouver ©M J Larkin 2008. PERCEPTIONS Jimi Hendrix ALL ALONG THE WATCHTOWER By Bob Dylan ©M J Larkin 2008.