* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Immunosuppressants_team2011-09
Discovery and development of integrase inhibitors wikipedia , lookup
5-HT3 antagonist wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Cell encapsulation wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Drug interaction wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
Prescription costs wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Neuropharmacology wikipedia , lookup
Ciclosporin wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Theralizumab wikipedia , lookup
Immunosuppressants
Prof. Alhaider, 1432 H
Definition
Clinical Uses (Organ transplants & Autoimmune
diseases)
Pre-requisite to understand immunosuppressive
drugs (Basic immunology).
Review of immune system (read Table 56-1,
next slide)
Cell mediated vs humoral immunity
Importance of cytokines
Immunophilins
Calcineurin
MOA: next slide
Classification of Immunosuppressant (Based on
Mechanism of Action)
A) Antiprolifirative Agents
I) Drugs Acting on Immunophilins: (most importanat )
a) Selective Inhibitors of Cytokine production (Calcineurin Inhibitors)
e.g: Cyclosporine; Tacrolimus
b) Inhibitor of cytokine function (e.g. Sirolimus).
II) Antimetabolites (Azathioprine; Mycophenolate Mofetil)
III ) Alkylating Agents (Cyclophosphamide)
1.
2.
B) Lymphocyte Depleting Agents:
Corticosteroids (Prednisone (orall) & Methylprednisolone (IV) )
Immunosuppressive Antibodies
a) Polyclonal Antibodies (Antilymphocytic Globulins)
b) Monoclonal Antibodies (Selective inhibitors of IL2 (Basiliximab; Daclizumab)
Immunosuppressant
Classes
Non-selective
Corticosteroids
Prednisone (PO) &
Methylprednisolone (IV)
Antimetabolite (DNA
synthesis inhibitors)
Azathioprine &
Myclophenolate mofetil
Immunoglobulins
Anti-lymphocyte
antibodies
Selective
Calcineurin Inhibitors
Cyclosporine
Tacrolimus (Rapamycin)
Selective IL-2 Receptor
antagonists
Basiliximab, Daclizumab &
Infliximab
Mamalian
target of
Rapamycin (mTOR)
inhibitors
Sirolimus
Selective Inhibitors of Cytokine Production
(Drugs Acting on Immunophilins)
1) Cyclosporine (CsA) (Cyclic peptide from Soil
Fungus (1971)
◦ MOA: (See slide 5)
CsA binds to cyclophillin forming a comlex Bind to
Calcineurin dephosphorylation NFATc * Synthesis of IL-2
cell-mediated immune response (proliferation of T cells)
Thus, decreases the level of IL-2, the primary chemical stimulus
for increasing the number of T lymphocytes.
Note: Suppress only cell-mediated immunity with no effect on B
lymphocytes/humoral immunity.
*NFATc = cytosolic nuclear factor of activated T cells
◦
PK
1. CsA available as oral (capsule) or parentral (i.v)
2. Oral bioavailability (20-50%),
3. It undergoes extensive hepatic metabolism by Cyt
P450 (CYP3A4) (Affected by drugs affecting the
microenzymes cimetidine, rifampin,
cefaporazone)
4. Mainly excreted in the bile.
◦ Clinical Uses of Cyclosporine:
1) Drug of choice for preventing organ transplant
rejection in combination with steroids and other
immunosuppressants.
2) Severe active rheumatoid arthritis, as an alternative
for methotrexate
3) Lower doses (7.5 mg/kg/d) for autoimmune
diseases (Uveitis; RA; early Rx of DM 1)
3) Psoriasis and asthma ? Not used anymore
◦ Side Effects (remember: most immunosuppressant are
very toxic)
Dose-dependent nephrotoxicity ( Risk of Rejection);
enhanced when given with other nephrotoxic drugs
(Aminoglycosides; NSIADs, amphotericin B)
Hepatotoxicity
Neurotoxicity as tremor and hallucination
Infection, opportunistic
Lymphoma and cancer How? immunity
Hypertension; hyperlipidemia; Hyperkalemia; DM;
osteoporosis, hirsutism, and gum hyperplasia (So What?
don’t prescribe it to female patints)
2. Tacrolimus (FK 506):
◦ More potent than Cyclosporine
◦ MOA: Similar to Cyclosporine (Calcineurin Antagonist)
but it bind to a different immunophilin (FKBP) slide 14
◦ PK: Almost Similar to Cyclosporine
◦ Side Effects: differ from Cyclosporine, that it has no
hirsutism or gum hyperplasia but may show more
hyperglycemia than cyclosporine.
◦ Note: It is like cyclosporine, could lead to nephrotoxicity
and hyperglycemia (more hyperglycemia than cyclosporine)
, and hyperlipidemia.
Clinical uses of Tacrolimus:
Preferred
over CsA for:
Its greater potency (50-100 times more potent than
cyclosporine)
lower rejection episodes
lower doses of glucocorticoids are used with lower
side effects
Better first choice for women, can you tell why? Less
hirsutism and gum hyperplasia
Severe refractory atopic dermatitis, local application
of an ointment
An ointment for psoriasis.
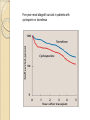
Five year renal allograft survival in patients with
cyclosporin or tacrolimus
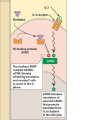
3. Sirolimus (Rapamycin Macrolides)
◦ MOA: see next slide
1) Binds to the same immunophilin Tacrolimus binds to
but does not form a complex with calcineurin. Instead, it
binds to mTOR (Mammalian Target of Rapamycin) which
is essential for many cellular functions (See Figure 40.6)
2) Sirolimus does not affect IL-2 production, unlike CsA
& Tac, rather inhibits T-cells response to IL-2 (Blocks
cytokine-stimulated cell proliferation)
3) Potent inhibitor of B-cell proliferation and
immunoglobulin production (decreases the humoral
immunity)
Uses of Sirolimus:
◦ 1) can be used together with cyclosporine to
increases the activity of cyclosporine for organ
transplantion. Why not tacrolimus? Because it shares
the same MOA.
◦ 2) As replacement of cyclosporine if transplanted
patient developed cancer of skin or lips.
◦ 3) Used in cardiac catheter stint to prevent stenosis??
◦ 4) As an ointment for atopic dermatitis and psoriasis
Side Effects:
◦ 1) Pneumonitis
◦ 2) Hyperlipidemia (even more than calcineurinantagonists)
Antiproliferatives (Continue…)
2. Antimetabolites (Cytotoxic Drugs)
◦ 1) Azathioprine: is a prodrug of mercaptopurine.
Cytotoxic, rarely used as chemotherapeutic drug, but
commonly used for immunosuppression.
It is a prodrug, converted in the body to the active
metabolite, 6-mercaptopurine and thioinosinic acid.
MOA: (see next slide Figure)
It inhibits purine synthesis (antimetabolite), thus interfering
with nucleic acid metabolism of leukocytes and lymphocytes
leading to the inhibition of proliferation.
Why it is considered as cytotoxic agent?
Because the purine analog of Azathioprine can destroy
lymphoids cells.
Why is it very important to know the structure of
azathioprine?.
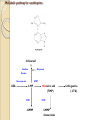
Metabolic pathway for azathioprine
6-thiouracil
(-)
Xanthine
Allopurinol
Oxidase
Nonenzymatic
AZA
HPRT
6-MP
TPMT
6-MMP
thioiosinic acid
(TIMP)
TPMT
6-MMP
ribonucleides
6-thioguanine
( 6-TG)
Cliniclal Uses of Azathioprine (ImuranR)
◦ 1) Maintenance of renal allograft and other transplants together
with steroids and cyclosporine.
◦ 2) Can be used for glomerulonephtitis and SLE; RA;
Crohn’s disease and multiple sclerosis (MS).
◦ PK:It can be given both orally and IV
Side Effects:
◦ 1) Like cyclosporine, is not teratogenic (Unlike TAC and Siro)
but carcinogenic if given together with alkylating agents. Here,
higher doses are used for immunosuppression as compared to its
use in autoimmune diseases.
◦ 2) Strong bone marrow suppression (Leukopenia; anemia;
thrombocytopenia How?
◦ 3) Hepatic dysfunction as increase ALP and mild jaundice.
◦ 4) Hypersensitivity reactions (as rashes, fever, diarrhea) Why?.
Drug
interactions:
Combination with ACEIs or
cotrimoxazole can cause severe
leukopenia in renal transplants
2) Mycophenolate Mofetil (CellceptR)
◦ The most important discovery among the
immunosuppressant agents.
◦ MOA: (See Figure in the coming 2nd slide)
Mycophenolic acid acts as non-competitive, selective 7 reversible
inhibitor of inosine monophosphate dehydrogenase (IMD)
Decreases GMP, which is a key enzyme in the de novo pathway of
purine synthesis. This leads to suppression of both B and T
lymphocyte activation.
PK: Good oral absorption;
◦ Side Effects:
Less than azathioprine, Reversible bone marrow suppresion
(leukopenia and anemia); N/V and diarrhea (decresed by Entericcoated form).
Unlike azathioprine it is teratogenic.
◦ Clinical Uses
1) Drug of choice for preventing vasculopathy in cardiac
transplants
2)As a replacement for the more cytotoxic drug,
azathioprine in renal allograft patients as well as liver,
heart et act. Why?
As replacement of azathioprine or cyclophosphamide for
autoimmune diseases (RA, SLE (especially before lupus
nephritis); Glomerulonephritis
Has good oral bioavailability
Note:
in renal or liver transplant, patients may
take the followings:
Corticosteriods as prednisolone (Low dose) +
cyclosporine or Tac + Mycophenolate Mofetil
◦ 3. Lefunomide:
- A Prodrug of an inhibitor of PYRIMIDINE synthesis rather
than purine-like azathioprine and mycophenolate mofetil.
◦ Orally active used only for RA
◦ Side effects: Alopecia; raised LFT, nephrotoxicity,
teratogenicity.
3. Alkylating Agents
e.g. Cyclophosphamide
◦ The most potent immunosuppressant
◦ Destroys proliferating lymphoid cells (cytotoxic agent) also
alkylates some resting cellsvery toxic
◦ Clinical Uses:
Before the discovery of Mycophenolate, cyclophosphamide was the
drug of choice for treatment of many autoimmune diseases like: SLE,
autoimmune hemolytic diseases, and RA.
◦ Side Effects:
Pancytopenia
Hemorrhagic cystitis a unique Adverse effect
Infertility
Teratogenic
B) Lymphocyte Depleting Agents:
1. Corticosteroids:
The most commonly used immunosuppressant
MOA:
◦ At the biochemical level: act on gene expression, which
leads to decrease synthesis of PGs; Leukotrienes, cytokines,
and other signaling molecules which participate in the
immune response.
◦ At the cellular level: they inhibit the proliferation of T
lymphocytes (cell mediated) and slightly dampen humoral
immunity (by increasing the catabolism of
immunoglobulins).
◦ At immunosuppressive doses, Corticosteroids are cytotoxic
and continuous uses causes lowering of IgGs.
Uses:
◦ In combination with other immunossppressants for
transplanted patients (To prepare the patients as
well as maintenance).
◦ To treat acute rejection episodes (high doses)
◦ To treat undesirable immunoreactions (to drugs or
asthma).
◦ For autoimmune diseases (idiopathic
thrompocytopenic purpura; inflammatory bowel
diease; rheumatoid arthritis; systemic lupus
erythematosus; and glomerulonephritis)
Adverse
effects:
Increased blood pressure How? By their aldostrone-like moiety
hyperglycemia due to increased gluconeogenesis, insulin resistance, and
impaired glucose tolerance ("steroid diabetes");
Osteoporosis
Visceral and truncal fat deposition (central obesity) and appetite stimulation
Weight gain (water & salt retention) How? Answered above!
Muscle breakdown (proteolysis), weakness; reduced muscle mass and repair
Increased skin fragility, easy bruising
Cataracts
Adrenal cortex suppression (Avoid abrupt withdrawl)
Increase tendency to infections
2. Immunosuppressive Antibodies
a) Polyclonal Antibodies (Antilymphocyte
Globulins)
Definition:Thymocytes are considered as T-cell precursors.
What are the differences between polyclonal and monoclonal antibodies?
1) Antithymocyte (Antilymphocyte) Globulins (ALG): this antisera can be obtained
by immunization of large animals (e.g.rabbits) with human lymphoid cells.
MOA: Antibodies bind to the surface of circulating T lymphocytes
forming a comlex. This complex will be phagocytosed in the liver or
spleen leading to destruction or inactivation of T cells.
ALG mainly affects the cellular immunity with no effect on humoral,
resulting in antibodies against these foreign proteins.
PK: Administered by IM or slow IV infusion with
long half-life of: 3-9 days
Side Effects:
1) Mainly result from the introduction of foreign
proteins obtained from heterogeneous serum
(Anaphylactic and serum sickness reactions;
Local pain and erythema at the site of injection).
2) Chills & fever and Leukopenia &
thrombocytopenia
3) Viral infections and skin rashes
4) Lymphoma and cancer
Polyclonal Antibodies (continue…)
Clinical Uses of ALG:
1) Rx of hyperacute phase of allograft rejection
2) To prepare the patient undergoing bone marrow
translpant (Give large doses of ALG for 7 days)
2) Immune globulin Intravenous (IGIV):
- Prepared from a pool of thousands of healthy donors.
Uses:
1) Refractory Idipathic Thrombocutopenic Purpura
Advantages: Has no antigenicity
B) Monoclonal Antibodies (Muromonab; Basiliximab;
Abciximab; Daclizumab.
1) Muromonab-CD3 (IL-2-antagonist):
From its name, it is murine monoclonal antibody prepared
by hybridoma technology and directed against the glycoprotien CD3
antigen of human T cells. Used mainly for cases of acute allograft
rejections of the kidney, heart, and liver.
It is also used to deplete T cells from donor bone marrow before
transplantation.
Advantage over ALG: More specific and T lymphocytes return to
normal within 24 hours.
Side Effects:
1) Cytokine release syndrome (Anaphylactoid reactions); and
seizure (contraindication)
Therefore, it is less used nowadays.
Side Effect of Muromonab
Its use has been declined much because of multiple side
effects and the emergence of newer and more selective
antibodies therapy
Anaphylaxis may occur
Cytokine release syndrome, flu-like to dangerous
shock-like reactions can occur, & high fever
CNS: Seizures, encephalopathy, cerebral
edema & headache
Infection like CMV
Contraindicated with pregnancy, breast
feeding, history of seizures, uncompensated
heart failure
2) Modified Types of monoclonal antibodies
(e.g: Selective Inhibitors of IL-2):
Note: Monoclonal Antibodies are not limited for
immunosuppression but could be utilized for other
purposes (See Table 56-3)
By using the genetic engineering, most murine amino
acids of Muromonab have been replaced by human
a.a.; producing humanized designated monoclonal
antibodies (e.g. Daclizumab; Transtuzumab). While
the chimeric (Mixed) antibodies contain XI in their
name (e.g. Abciximab; Infliximab; Rutuximab).
Clinical Uses: See Table 56-3
Advantages over polyclonal antibodies Are less
immunoantigenic.
B- Selective IL-2 Receptor Antagonists
Basiliximab & Daclizumab
Basiliximab is a chimeric antibody composed
of 25% murine & 75% human protein. Blocks
IL-2 of activated lymphocytes
Daclizumab is humanized antibody composed
of 90% human protein
Therapeutic Use:
Prophylaxis against acute rejection of kidney
transplantation
Used in combination with steroids and CsA
Selective IL-2 Receptor Antagonists
Basiliximab & Daclizumab (Continue…)
Mechanism of action:
They are anti-CD25 and IL-2 antibodies
They bind to the -chain of the IL-2 R (CD25
or TAC subunit) on the activated T-cells
Then, IL-2 binding to IL-2R is prohibited & T-cell
activation and proliferation are suppressed
VI- Selective IL-2 Receptor Antagonists
Basiliximab & Daclizumab (Continue...)
Pharmacokinetics: Given by IV route
Daclizumab has serum half-life of 20 days &
receptor blockade for 120 days
Administered in 5 doses; the first 24 hours
before transplantation and next 4 doses at 14days intervals
Basiliximab has serum half-life of 7 days
Administered in two doses; the first at 2-hours
before transplantation & the second at 4 days
after surgery
Selective IL-2 Receptor Antagonists
Basiliximab & Daclizumab )Contiue..)
Adverse Effects:
Both are well-tolerated
Gastrointestinal toxicity is the major one
NO antibodies, of clinical relevance, to
the antibodies are produced
Infection & malignancy were not reported
Alemtuzumab:
Humanized monoclonal antibody directed
against CD-52, and produces profound
depletion of T cells.
Used for refractory B-cell chronic
lymphocytic leukemia. However, it is
currently used in organ transplantation.
3- RhoD Immunoglobulin
Rho(D) Immune Globulin (Rhogam)
Rhogam
is an immunoglobulin that recognizes the
Rho(D) antigen
Prepared from pooled sera from Rho-negative
volunteers immunized with D+ erythrocytes
It prevents erythroblastosis fetalis (hemolytic disease
of the newborn)
When a Rho(D)-negative mother carries a Rho(D)positive fetus, the mother becomes sensitized
Subsequent pregnancies can strengthen response
increasing chance of Ab transfer to the fetus in the last
trimester
How Does Rho(immunoglobulin
work)
The potion is a solution of IgG anti-D (antiRhD) antibodies that bind to, and lead to the
destruction of, fetal Rh D positive red blood
cells that have passed from the fetal circulation to
the maternal circulation. Therefore, Rho in a
Rhesus negative mother it can prevent
sensitization of the maternal immune system to
Rh D antigens, which can cause rhesus disease in
the current or in subsequent pregnancies. Note:
With the widespread use of Rho (D) Immune Globulin, Rh
disease of the fetus and newborn has almost disappeared.
RhoD Immunoglobulin
It is usually given to the mother within 72
hours after the birth of any of the Rhpositive babies she has.
This would prevent hemolytic anemia that
may occur in subsequent pregnancies
Adverse Effects:
Chills
Fever
Anaphylaxis (rare)