* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Fig.1 NEW PARADIGM HAS FOUR MAJOR THEMES (I)
Survey
Document related concepts
Transcriptional regulation wikipedia , lookup
Protein moonlighting wikipedia , lookup
Genome evolution wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Community fingerprinting wikipedia , lookup
Molecular evolution wikipedia , lookup
List of types of proteins wikipedia , lookup
Gene expression wikipedia , lookup
Expression vector wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Metabolomics wikipedia , lookup
Gene expression profiling wikipedia , lookup
Transcript
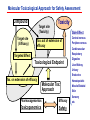
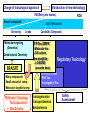
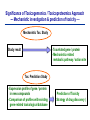
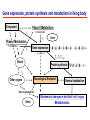
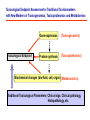
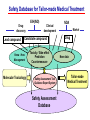
Toxicogenomics in the USA - Molecular toxicological approach in drug discovery and development - Current use of toxicogenomics in preclinical studies under assessing the current limitations and future promise Pfizer, Global Research & Development Drug Safety Evaluation Ikuo HORII Disease is the outcome of the interaction between genes and environment Genes Disease Environment Diet Organs Gene related Factor Life-style Medicines Most toxicologically relevant outcomes require differential expression of multiple genes Toxicity Side Effect directly caused by DNA damage Critical !!! (Mutagenicity, Carcinogenicity, etc.) Toxicity indirectly derived from the changes of related gene expression Manageable ! Molecular Tox. Approach Toxicopanomics Toxicogenomics Toxicoproteomics Metabolomics Traditional Toxicology for Safety Assessment Whole body assessment Regulatory Tox. Single Admin. Tox. Repeated Admin. Tox. Reproduction Tox. Carcinogeneicity Gene Tox. Specific Tox. etc.. Investigative Tox. - General observation (Lethality, Clinical sign, …) - Body weight, Food consumption - Clinical Pathology (Blood chemistry, Hematology, Urinalysis, …) - Functional assessment (Hepatic/Renal, CV, …) - Histopathology (Organ wt., Morphology, …) In Vitro Alternative Molecular Toxicology - New Science - New Technology Gene expression and toxicology Most toxicologically relevant outcomes require differential expression of multiple genes. If toxicity manifested at the level of organism is preceded by altered expression of related genes, its detection can serve as an early warning for subsequent deleterious outcomes Miniaturization and automation of new tools for analysis of gene expression and metabolic networking allow the molecular life of cells to be studied at a more holistic (and complex) level than was previously possible Molecular Toxicological Approach for Safety Assessment Compound Target site ( Efficacy) Target site (Toxicity) Toxicity Side Effect Tox. out of extension of efficacy Central nervous Peripher.nervous Cardiovascular Respiratory Targeted Effect Toxicological Endpoint Digestive Liver/Kidney Urinary Endocrine Tox. on extension of efficacy Molecular Tox. Approach Hematopoietic Muscle/Skeletal Skin Sensory Pharmacogenomics Efficacy Toxicopanomics Safety etc. Change of toxicological approach Introduction of new technology IND(Entry into human) Nos of Compound Screening NDA Cost / Resources Leads Molecular-targeting (Genomics) Combinatorial Chemistry SRA/SRT • Many compounds • Small amount of comp. Candidate Compound HTP-Tox./DMPK - Molecular tox. - Cell culture New analytics Regulatory Toxicology - LC/MS/MS (cassette dose) HTP-Tox. in vivo Pilot Tox. Investigative Tox…. • Molecular-targeted comp. Molecular Toxicology Toxicopanomics + New Science Toxicogenomics Toxicoproteomics Metabolomics Safety Assessment Study Design for Toxicogenomics/Toxicoproteomics Assessment Detection - Gene expression : Gene chip analysis, etc - Protein synthesis : 2-D Electrophoresis, Protein analysis Study Design (Comparison with known toxic-compounds under the database) In vivo In vitro Normal(non-treat) & Treated Cell / Organ / Tissue - Non - change - Up - regulation - Down - regulation Data Analysis Toxicity-related Gene Database Archives-database (Published information) Significance of Toxicogemonics / Toxicoproteomics Approach --- Mechanistic investigation & prediction of toxicity --Mechanistic Tox. Study Study result - Tox.related gene / protein - Mechanistic related metabolic pathway / action site Tox. Prediction Study - Expression profile of gene / protein in new compounds - Comparison of profiles with existing gene-related toxicological database Prediction of Toxicity ( Strategy of drug discovery ) Genes on the toxicology gene chip Functional group Stress response Cell proliferation Apoptosis DNA damage Inflammation Oxidative stress Drug metabolism Transporter Type of genes Oncogenes Acute phase response Signal transduction Transcription factors Cell cycle regulation Growth factors and receptor Tumor suppressors Caspases Apoptic regulators DNA repair DNA morphology Cytokines Vasoregulators, etc. Glutathione metabolism Oxidase Protein thioles Cytochrome P450s Glutathione transferase UGT Organic Peptide Ion pumps Toxicogenomics・Toxicoproteomics・Metabolomics DNA : Genome (Genomics) Gene-polymorphism (SNPs etc) RNA : Transcriptome (Transcriptomics) Gene expression profile (mRNA ) Proteome (Proteomics) Protein synthesis profile (Molecular function) Protein : Biochemicals : Metabolome (Metabolites) (Metabolomics) Metabolite-pattern profile (Urine, etc) Timing of gene expression and protein synthesis --- Toxicological assessment point ? --Toxicological stimulation(Trigger) DNA Signal mRNA mRNA Level Toxicogenomics Appearance of toxicity Protein Protein Level Toxicoproteomics Metabolic pattern in organism --- Process from toxicity appearance through damage to restoration --- Cell injury 20 Metabolic change in injury site z 10 Outbreak of injury 0 10 y Repair of injury 10 x 20 0 liver (steatosis) Toxicity type & site heart Pattern recognition renal medulla renal cortex control - Combination of changes - Severity Pattern analysis Database Gene expression, protein synthesis and metabolism in living body Compound Phase II Metabolism m metabolites t1 Phase I Metabolism n metabolites t2 t3 Gene expression Liver t1g1, t2g1, t3g1, t1g2, t2g2, t3g2, …...t1gi, t2gi, t3gFn,m up or down T1, T2, T3,etc Blood Protein synthesis Ti(t1p1,t2p1,t3p1….tipi ) tx Toxicological Endpoint Other organs General metabolism Gene regulations etc. Biochemical changes in bio-fluid / cell / organ Urine Metabolomics Toxicological Endpoint Assessment in Traditional Tox-biomarkers with New Markers of Toxicogenomics, Toxicoproteomics and Metabolomics Toxicological Endpoint Gene expression (Toxicogenomics) Protein synthesis (Toxicoproteomics) Biochemical changes (bio-fluid, cell, organ) (Metabonomics) Traditional Toxicological Parameters:Clinical sign, Clinical pathology, Histopathology, etc. Safety Database for Tailor-made Medical Treatment EIH(IND) Drug discovery Lead compound Clinical development Candidate compound Know - How Management Molecular Toxicology Toxicity / Side-effect Prediction Countermeasure Safety Assessment Tool Guidance Expert System Safety Assessment Database NDA Market Drug New data Tailor-made Medical Treatment The 2002 Workshop on Pharmacogenetics/Pharmacogenomics in Drug Development and Regulatory Decision-Making --- Sponsored jointly the FDA, DruSafe PhRMA and PWG --May 16 - 17, 2002 at the University of Maryland, Shady Grove Conference Center Toxicogenomics in Drug Development : Where are we today & where are we going ? Industry and regulatory agencies viewed this meeting as an opportunity to discuss how such data should be included/evaluated in IND and NDA applications. Where are we now ? Where would we like to be ? (1) Is toxicogenomic science and validation technology sufficiently mature to reply upon genomic data for safety decisions and to justify the routine use of genomic data in GLP toxicology studies ? Current toxicogenomic data is not being collected in GLP studies, and the data is difficult to interpret and do not add to standard toxicology assays. However, genomic data is useful in mechanistic studies, and if done with IND compounds, the data should be submitted. There was some consensus that genomic data may be added to standard toxicology data, but we need to explore the “safe harbor” concept with FDA. (2) Where is the value of toxicogenomic data to Industry and the FDA ? The value of toxicogenomic data now is in mechanistic studies and hypothesis testing and not predictive data in risk assessment. Most would like to develop more confidence in data, share data with FDA. (3) How could data from genomic arrays, in conjunction with standard short-term toxicology studies, be used to assist in study design or in species selection for long-term toxicology studies ? The toxicogenomics is not well understood presently to be predictive, especially outside the rat/mouse species, of the human response. The standard toxicology studies need not include or be replaced by genomics, but genomic data may be used to better design of toxicology. (4) Is there a need for guidance in the toxicogenomics area ? If guidance’s existed what wold be their main purpose and what would be the potential impact ? A regulatory guidance document is not necessary at this time. However, standard practice for reviewing data needs to be made transparent and a consensus of how data should be submitted would be useful. Thus, a white paper on how to review genomic data, within FDA, for internal consistency is recommended. (5) Development of “historic databases” in interpreting toxicogenomic findings may be useful if the data are robust and reliable and if toxicogenomics profiles predict toxicities. If this is correct, how should such databases be developed and utilized ? The development of some form of knowledge base rather than a historical database for interpreting toxicogenomic findings. Since the technology is emerging, and data is limited, the potential of genomic data is a “red flag” awareness to evaluate in other toxicological assays. As a conclusion, The application of toxicogenomics disciplines ranges from hypothesis testing of toxicity to safety evaluation. However, validation of the results for use in registration and marketing is limited and can only be evaluated on a case by case basis at the present time. As we progress, the regulatory implications of toxicogenomic data will be transparent and lead to relevant guidance documents.